Pinobanksin
Pinobanksin
 |
| Names |
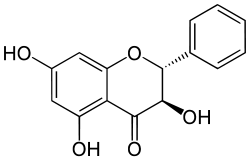
| IUPAC name
(2S,3R)-3,5,7-Trihydroxy-2-phenyl-chroman-4-one |
| Other names
3,5,7-trihydroxyflavanone, 3,5,7-trihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one |
| Identifiers |
| |
548-82-3  N N |
| 3D model (Jmol) |
Interactive image |
| ChEBI |
CHEBI:28103  N N |
| ChEMBL |
ChEMBL608410  Y Y |
| ChemSpider |
65962  Y Y |
| PubChem |
73202 |
InChI=1S/C15H12O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,14-17,19H/t14-,15+/m0/s1  Y YKey: SUYJZKRQHBQNCA-LSDHHAIUSA-N  Y YInChI=1/C15H12O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,14-17,19H/t14-,15+/m0/s1 Key: SUYJZKRQHBQNCA-LSDHHAIUBG
|
O=C2c3c(O[C@H](c1ccccc1)[C@H]2O)cc(O)cc3O
|
| Properties |
| |
C15H12O5 |
| Molar mass |
272.25 g/mol |
| Density |
1.497 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?) |
| Infobox references |
|
|
Pinobanksin is an antioxidant bioflavonoid (specifically a dihydroflavonol, a category of flavonol) that inhibits peroxidation of low density lipoprotein and it has electron donor properties reducing alpha-tocopherol radicals. It is present in sunflower honey.[1]
Pinobanksin is biosynthesized from pinocembrin.
References
- ↑ Sabatier, S.; Amiot, M.J.; Tacchini, M.; Aubert, S. (1992). "Identification of Flavonoids in Sunflower Honey". Journal of Food Science. 57 (3): 773. doi:10.1111/j.1365-2621.1992.tb08094.x.
Flavonols and their conjugates |
|---|
|
| Backbone | |
|---|
|
| Flavonols | Aglycones | |
|---|
| Conjugates | | |
|---|
| |
- Afzelin (Kaempferol 3-rhamnoside)
- Astragalin (kaempferol 3-O-glucoside)
- Kaempferitrin (kaempferol 3,7-dirhamnoside)
- Juglanin (Kaempferol 3-O-arabinoside)
- Kaempferol 3-alpha-L-arabinopyranoside
- Kaempferol 3-alpha-D-arabinopyranoside
- Kaempferol 7-alpha-L-arabinoside
- Kaempferol 7-O-glucoside
- Kaempferol 3-lathyroside
- Kaempferol 4'-rhamnoside
- Kaempferol 5-rhamnoside
- Kaempferol 7-rhamnoside
- Kaempferol 7-O-alpha-L-rhamnofuranoside
- Kaempferol 3-xyloside
- Kaempferol 7-xyloside
- Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)
- Kaempferol 3-O-rutinoside
- Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
- Trifolin (Kaempferol 3-O-beta-D-galactoside)
|
|---|
| | |
|---|
| | |
|---|
|
|---|
|
|---|
|
| O-Methylated flavonols | Aglycones | |
|---|
| Glycosides | of isorhamnetin |
- Narcissin (Isorhamnetin 3-O-rutinoside)
- Isorhamnetin 3-O-glucoside
- Tamarixetin 7-rutinoside
|
|---|
| other |
- Azalein (Azaleatin 3-O-α-L-rhamnoside)
- Centaurein (Centaureidin 7-O-glucoside)
- Eupalin (Eupalitin 3-0-rhamnoside)
- Eupatolin (Eupatolitin 3-O-rhamnoside)
- Jacein (Jaceidin 7-O-glucoside)
- Patulitrin (Patuletin 7-O-glucoside
- Xanthorhamnin (Rhamnetin glycoside)
|
|---|
|
|---|
|
|---|
|
| Derivative flavonols | Aglycones |
- Noricaritin
- Dihydronoricaritin
|
|---|
| Glycosides | |
|---|
|
|---|
|
| Pyranoflavonols | |
|---|
|
| Furanoflavonols | |
|---|
|
| Semisynthetic | |
|---|
