Atrimustine
 | |
| Identifiers | |
|---|---|
| |
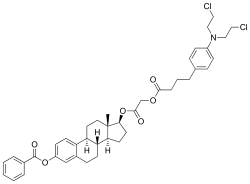
| Synonyms | 3-Benzoyl-17-((4-(4-(bis(2-chloroethyl)amino)phenyl)-1-oxobutoxy)acetyl-17β-estradiol[1] |
| CAS Number | 75219-46-4 |
| PubChem (CID) | 6917688 |
| ChemSpider | 5292918 |
| UNII | XC0K09B7K4 |
| ChEMBL | CHEMBL2106381 |
| Chemical and physical data | |
| Formula | C41H47Cl2NO6 |
| Molar mass | 720.73 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Atrimustine (INN) (developmental code name KM-2210), also known as bestrabucil or busramustine, is a nitrogen mustard alkylating antineoplastic drug that was under development in Japan by Kureha Chemicals (now Kureha Corporation) for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients.[1][2] It is the benzoate ester of a conjugate of estradiol and chlorambucil,[3] which results in targeted/site-directed DNA alkylating activity toward estrogen receptor-positive tissues such as breast and bone.[4][5] It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.[1]
See also
References
- 1 2 3 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–898. ISBN 978-1-4757-2085-3.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 438–. ISBN 978-0-8155-1856-3.
- ↑ The Cancer Bulletin. Medical Arts Publishing Foundation. 1987. p. 245.
- ↑ Ohsawa N, Yamazaki Z, Wagatsuma T, Isurugi K (1984). "[Bestrabacil: a possible target-oriented anticancer agent]". Gan To Kagaku Ryoho (in Japanese). 11 (10): 2115–24. PMID 6548354.
- ↑ Joji Ishigami (1985). Recent Advances in Chemotherapy: Proceedings of the 14th Internat. Congress of Chemotherapy, Kyoto, 1985. Antimicrobial section ; 1. 1 ,1. University of Tokyo Press. p. 52,54,471. ISBN 978-0-86008-385-6.