Met-enkephalin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[[(2S)-2-[[2-[[2-[[(2S)-2-Smino-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-4-methylsulfanylbutanoic acid | |
| Other names
[Methionine]enkephalin; L-Tyrosylglycylglycyl-L-phenylalanyl-L-methionine | |
| Identifiers | |
| 58569-55-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL13786 |
| ChemSpider | 391597 |
| ECHA InfoCard | 100.055.741 |
| EC Number | 261-335-8 |
| 1614 | |
| KEGG | C11684 |
| MeSH | Enkephalin,+Methionine |
| PubChem | 443363 |
| |
| |
| Properties | |
| C27H35N5O7S | |
| Molar mass | 573.67 g·mol−1 |
| log P | 0.607 |
| Acidity (pKa) | 3.234 |
| Basicity (pKb) | 10.763 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF),[1] is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.[2]
History
Met-enkephalin was discovered and characterized by Hughes, Kosterlitz, et al. in 1975 after a search for endogenous ligands of the opioid receptors.[3]
Chemistry
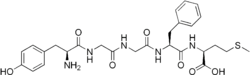
Met-enkephalin is a pentapeptide with the amino acid sequence tyr-gly-gly-phe-met. The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine.
Pharmacology
Distribution
Met-enkephalin is found mainly in the adrenal medulla and throughout the central nervous system (CNS), including in the striatum, cerebral cortex, olfactory tubercle, hippocampus, septum, thalamus, and periaqueductal gray, as well as the dorsal horn of the spinal cord.[2] It is also present in the periphery, notably in some primary afferent fibers that innervate the pelvic viscera.[2]
Biosynthesis
Met-enkephalin is synthesized from proenkephalin via proteolyic cleavage[4] in two metabolic steps. Proenkephalin A is first reduced by either one of two trypsin-like endopeptidase enzymes, prohormone convertase 1 (PC1) or prohormone convertase 2 (PC2); then, the resulting intermediates are further reduced by the enzyme carboxypeptidase E (CPE; previously known as enkephalin convertase (EC)).[5][6] Proenkephalin A contains four sequences of met-enkephalin (at the following positions: 100–104; 107–111; 136–140; 210–214), and as a result, its cleavage generates four copies of met-enkephalin peptides at once.[4] In addition, anabolism of proenkephalin A results in the production of one copy each of two C-terminal-extended met-enkephalin derivatives, the heptapeptide met-enkephalin-arg-phe (261–267), and the octapeptide met-enkephalin-arg-gly-leu (186–193),[4] though whether they affect the opioid receptors in a similar manner as met-enkephalin is not entirely clear.[7]
Clearance
Met- and leu-enkephalin are metabolized by a variety of different enzymes, including aminopeptidase N (APN),[8] neutral endopeptidase (NEP),[8] dipeptidyl peptidase 3 (DPP3),[8] carboxypeptidase A6 (CPA6),[9] and angiotensin-converting enzyme (ACE).[10] These enzymes are sometimes referred to as enkephalinases.
Pharmacodynamics
Met-enkephalin is a potent agonist of the δ-opioid receptor, and to a lesser extent the μ-opioid receptor, with little to no effect on the κ-opioid receptor. It is through these receptors that met-enkephalin produces its opioid effects, such as analgesia and antidepressant-like effects.
It is also the endogenous ligand of the opioid growth factor receptor (OGFR; formerly known as the ζ-opioid receptor), which plays a role in the regulation of tissue growth and regeneration; hence why met-enkephalin is sometimes called OGF instead.
Pharmacokinetics
Met-enkephalin has low bioavailability, is rapidly metabolized, and has a very short half-life (minutes).[3][11] These properties are considered undesirable in pharmaceuticals as large doses would need to be administered multiple times an hour to maintain a therapeutically relevant effect, making it unlikely that met-enkephalin will ever be used as a medicine.
[D-Ala2]-Met-enkephalinamide (DALA), is a synthetic enkephalin analog which is not susceptible to degradation by brain enzymes and at low doses (5 to 10 micrograms) caused profound, long-lasting, morphine-like analgesia when microinjected into rat brain.[12]
See also
References
- ↑ Zagon IS, Isayama T, McLaughlin PJ (January 1994). "Preproenkephalin mRNA expression in the developing and adult rat brain". Brain Research. Molecular Brain Research. 21 (1-2): 85–98. doi:10.1016/0169-328x(94)90381-6. PMID 8164525.
- 1 2 3 Christoph Stein (1999). = 4Rfr8cQayvgC&pg = PA22 Opioids in pain control: basic and clinical aspects Check
|url=value (help). Cambridge University Press. pp. 22–23. ISBN 978-0-521-62269-1. Retrieved 25 November 2011. - 1 2 Thomas Carleton Moore (1993). = dJyJGevjkzQC&pg = PA179 Neurovascular immunology: vasoactive neurotransmitters and modulators in cellular immunity and memory Check
|url=value (help). CRC Press. p. 179. ISBN 978-0-8493-6894-3. Retrieved 25 November 2011. - 1 2 3 Fleur L. Strand (1999). = PNPU0sknfCUC&pg = PA348 Neuropeptides: regulators of physiological processes Check
|url=value (help). MIT Press. p. 348. ISBN 978-0-262-19407-5. Retrieved 25 November 2011. - ↑ Costa E, Mocchetti I, Supattapone S, Snyder SH (July 1987). "Opioid peptide biosynthesis: enzymatic selectivity and regulatory mechanisms". The FASEB Journal. 1 (1): 16–21. PMID 3111927.
- ↑ Krajnik M, Schäfer M, Sobanski P, et al. (May 2010). "Enkephalin, its precursor, processing enzymes, and receptor as part of a local opioid network throughout the respiratory system of lung cancer patients". Human Pathology. 41 (5): 632–42. doi:10.1016/j.humpath.2009.08.025. PMID 20040394.
- ↑ Vats ID, Chaudhary S, Karar J, Nath M, Pasha Q, Pasha S (October 2009). "Endogenous peptide: Met-enkephalin-Arg-Phe, differently regulate expression of opioid receptors on chronic treatment". Neuropeptides. 43 (5): 355–62. doi:10.1016/j.npep.2009.07.003. PMID 19716174.
- 1 2 3 Thanawala V, Kadam VJ, Ghosh R (October 2008). "Enkephalinase inhibitors: potential agents for the management of pain". Current Drug Targets. 9 (10): 887–94. doi:10.2174/138945008785909356. PMID 18855623.
- ↑ Lyons PJ, Callaway MB, Fricker LD (March 2008). "Characterization of carboxypeptidase A6, an extracellular matrix peptidase". The Journal of Biological Chemistry. 283 (11): 7054–63. doi:10.1074/jbc.M707680200. PMID 18178555.
- ↑ Benuck M, Berg MJ, Marks N (1982). "Separate metabolic pathways for Leu-enkephalin and Met-enkephalin-Arg(6)-Phe(7) degradation by rat striatal synaptosomal membranes". Neurochemistry International. 4 (5): 389–96. doi:10.1016/0197-0186(82)90081-X. PMID 20487892.
- ↑ William J. Kraemer; Alan David Rogol (29 August 2005). The endocrine system in sports and exercise. John Wiley & Sons. pp. 203–. ISBN 978-1-4051-3017-2. Retrieved 25 November 2011.
- ↑ Pert, C. B.; Pert, A.; Chang, J. K.; Fong, B. T. (1976-10-15). "(D-Ala2)-Met-enkephalinamide: a potent, long-lasting synthetic pentapeptide analgesic". Science (New York, N.Y.). 194 (4262): 330–332. ISSN 0036-8075. PMID 968485.