Fluoroantimonic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Fluoroantimonic acid | |
| Systematic IUPAC name
Fluoranium hexafluorostibanuide Fluoranium hexafluoridoantimonate(1−) | |
| Identifiers | |
| 16950-06-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 32741664 |
| ECHA InfoCard | 100.037.279 |
| EC Number | 241-023-8 |
| |
| |
| Properties | |
| H 2SbF 7 | |
| Molar mass | 256.765 |
| Appearance | Colorless liquid |
| Density | 2.885 g/cm3 |
| Solubility | SO2ClF, SO2 |
| Acidity (pKa) | −25 |
| Basicity (pKb) | 39 |
| Hazards | |
| Main hazards | Extremely corrosive, Violent hydrolysis |
| H300, H310, H314, H330, H411 | |
| P260, P264, P273, P280, P284, P301+310 | |
| R-phrases | R26, R29, R35 |
| S-phrases | (S1/2), S36/37/39, S45, S53, S60, S61 |
| NFPA 704 | |
| Related compounds | |
| Related acids |
Antimony pentafluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
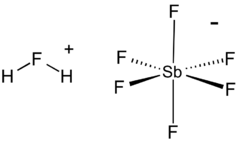
Fluoroantimonic acid (systematically named fluoranium hexafluorostibanuide and fluoranium hexafluoridoantimonate(1−)) is an inorganic compound with the chemical formula H
2FSbF
6 (also written H
2F[SbF
6], 2HF·SbF5, or simply HF-SbF5). It is an ionic liquid created by reacting hydrogen fluoride (HF) with antimony pentafluoride (SbF5) in a stoichiometric ratio of 2:1. It is the strongest known superacid, which has been demonstrated to protonate even hydrocarbons to afford pentacoordinate carbocations (which decompose quickly in regular carbocations and H2).[1] Similar acids can be created by using excess antimony pentafluoride.[2]
The reaction to produce fluoroantimonic acid is:
- 2 HF ⇌ H2F+ + F−
- SbF5 + F− → SbF−
6
The overall reaction is:
- SbF5 + 2 HF → SbF−
6 + H2F+
The second reaction is not in equilibrium, therefore the overall reaction is not in equilibrium. The reaction is exothermic.
The F−, produced by autoionization of hydrogen fluoride, reacts with SbF5 to yield the adduct SbF−
6. In the fluoronium ion, hydrogen fluoride is coordinated to the hydrogen ion. The anion is classified as noncoordinating and is both a very weak nucleophile and a very weak base.
The acid is often said to contain "naked protons", but the "free" protons are, in fact, always bonded to hydrogen fluoride molecules to make the fluoronium cations (similar to the hydronium cation in aqueous solution).[3] It is the fluoronium ion that accounts for fluoroantimonic acid's extreme acidity. Fluoroantimonic acid is 1016 (10 quadrillion) times stronger than 100% sulfuric acid.[4] The protons easily migrate through the solution, moving from H2F+ to HF, when present, by the Grotthuss mechanism:
- H2F+ + HF ⇌ HF + H2F+
Fluoroantimonic acid thermally decomposes at higher temperatures, generating hydrogen fluoride gas.
Structure
Two related products have been crystallised from HF-SbF5 mixtures, and both have been analyzed by single crystal X-ray crystallography. These salts have the formulas [H
2F+
][Sb
2F−
11] and [H
3F+
2][Sb
2F−
11]. In both salts, the anion is Sb
2F−
11.[5] As mentioned above, SbF−
6 is weakly basic; the larger anion Sb
2F−
11 is expected to be still weaker.
The following values are based upon the Hammett acidity function. Increased acidity is indicated by smaller (in this case, more negative) values of H0.
- Fluoroantimonic acid (1990) (H0 value = −31.3)
- Magic acid (1974) (H0 value = −19.2)
- Carborane acid (1969) (H0 value = −18.0)
- Fluorosulfuric acid (1944) (H0 value = −15.1)
- Triflic acid (1940) (H0 value = −14.9)
Applications
This extraordinarily strong acid protonates nearly all organic compounds. In 1967, Bickel and Hogeveen showed that 2HF·SbF5 will remove H2 from isobutane and methane from neopentane to form carbenium ions:[6][7]
- (CH3)3CH + H+ → (CH3)3C+ + H2
- (CH3)4C + H+ → (CH3)3C+ + CH4
Materials compatible with fluoroantimonic acid as a solvent include SO2ClF, and sulfur dioxide; some chlorofluorocarbons have also been used. Containers for HF-SbF5 are made of PTFE.
Safety
HF-SbF5 is extremely corrosive, toxic, and moisture sensitive.[2] Like most strong acids, fluoroantimonic acid can react violently with water, owing to the exothermic hydration. Consequently, it cannot be used in aqueous solution, only in hydrofluoric solution.
See also
References
- ↑ Olah, G. A. (2001). A Life of Magic Chemistry: Autobiographical Reflections of a Nobel Prize Winner. John Wiley and Sons. pp. 100–101. ISBN 0-471-15743-0.
- 1 2 Olah, G. A.; Prakash, G. K. Surya; Wang, Qi; Li, Xing-ya (15 April 2001). "Hydrogen Fluoride–Antimony(V) Fluoride". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley and Sons. doi:10.1002/047084289X.rh037m. ISBN 9780470842898.
- ↑ Klein, Michael L. (October 25, 2000). "Getting the Jump on Superacids" (pdf). Pittsburgh Supercomputing Center (PSC). Retrieved 2012-04-15.
- ↑ Olah, G. A. (2005). "Crossing Conventional Boundaries in Half a Century of Research". Journal of Organic Chemistry. 70 (7): 2413–2429. doi:10.1021/jo040285o. PMID 15787527.
- ↑ Mootz, Dietrich; Bartmann, Klemens (March 1988). "The Fluoronium Ions H2F+ and H
3F+
2: Characterization by Crystal Structure Analysis". Angewandte Chemie International Edition. 27 (3): 391–392. doi:10.1002/anie.198803911. - ↑ Bickel, A. F.; Gaasbeek, C. J.; Hogeveen, H.; Oelderik, J. M.; Platteeuw, J. C. (1967). "Chemistry and spectroscopy in strongly acidic solutions: reversible reaction between aliphatic carbonium ions and hydrogen". Chemical Communications. 1967 (13): 634–635. doi:10.1039/C19670000634.
- ↑ Hogeveen, H.; Bickel, A. F. (1967). "Chemistry and spectroscopy in strongly acidic solutions: electrophilic substitution at alkane-carbon by protons". Chemical Communications. 1967 (13): 635–636. doi:10.1039/C19670000635.
