Xenon difluoride
| | |
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Xenon difluoride Xenon(II) fluoride | |
| Identifiers | |
| 13709-36-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 75497 |
| ECHA InfoCard | 100.033.850 |
| PubChem | 83674 |
| |
| |
| Properties | |
| F2Xe | |
| Molar mass | 169.29 g·mol−1 |
| Appearance | White solid |
| Density | 4.32 g/cm3, solid |
| Melting point | 128.6 °C (263.5 °F; 401.8 K)[1] |
| 25 g/l (0 °C) | |
| Vapor pressure | 6.0×102 Pa[2] |
| Structure | |
| parallel linear XeF2 units | |
| Linear | |
| 0 D | |
| Thermochemistry | |
| Std molar entropy (S |
254 J·mol−1·K−1[3] |
| Std enthalpy of formation (ΔfH |
−108 kJ·mol−1[3] |
| Hazards | |
| Main hazards | Corrosive to exposed tissues. Releases toxic compounds on contact with moisture.[4] |
| Safety data sheet | PELCHEM MSDS |
| NFPA 704 | |
| Related compounds | |
| Other anions |
Xenon dichloride |
| Other cations |
Krypton difluoride Radon difluoride |
| Related compounds |
Xenon tetrafluoride Xenon hexafluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with light or water vapor. Xenon difluoride is a dense, white crystalline solid. It has a nauseating odour and low vapor pressure.[5]
Structure
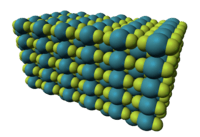
Xenon difluoride is a linear molecule with an Xe–F bond length of 197.73±0.15 pm in the vapor stage, and 200 pm in the solid phase. The packing arrangement in solid XeF
2 shows that the fluorine atoms of neighbouring molecules avoid the equatorial region of each XeF
2 molecule. This agrees with the prediction of VSEPR theory, which predicts that there are 3 pairs of non-bonding electrons around the equatorial region of the xenon atom.[2]
At high pressures, novel, non-molecular forms of xenon difluoride can be obtained. Under a pressure of ~50 GPa, XeF
2 transforms into a semiconductor consisting of XeF
4 units linked in a two-dimensional structure, like graphite. At even higher pressures, above 70 GPa, it becomes metallic, forming a three-dimensional structure containing XeF
8 units.[6] However, a recent theoretical study has put these experimental results in doubt.[7]
Chemistry
Synthesis
Synthesis proceeds by the simple reaction:
- Xe + F2 → XeF2
The reaction needs heat, irradiation, or an electrical discharge. The product is a solid. It is purified by fractional distillation or selective condensation using a vacuum line.[8]
The first published report of XeF2 was in October 1962 by Chernick, et al.[9] However, though published later,[10] XeF2 was probably first created by Rudolf Hoppe at the University of Münster, Germany, in early 1962, by reacting fluorine and xenon gas mixtures in an electrical discharge.[11] Shortly after these reports, Weeks, Cherwick, and Matheson of Argonne National Laboratory reported the synthesis of XeF2 using an all-nickel system with transparent alumina windows, in which equal parts Xe and F2 gases react at low pressure upon irradiation by an ultraviolet source to give XeF2.[12] Williamson reported that the reaction works equally well at atmospheric pressure in a dry Pyrex glass bulb using sunlight as a source. It was noted that the synthesis worked even on cloudy days.[13]
In the previous syntheses the F2 reactant had been purified to remove HF. Šmalc and Lutar found that if this step is skipped the reaction rate proceeds at four times the original rate.[14]
In 1965, it was also synthesized by reacting xenon gas with dioxygen difluoride.[15]
Solubility
XeF
2 is soluble in solvents such as BrF
5, BrF
3, IF
5, anhydrous HF, and CH
3CN, without reduction or oxidation. Solubility in HF is high, at 167g per 100g HF at 29.95 °C.[2]
Derived xenon compounds
Other xenon compounds may be derived from xenon difluoride. The unstable organoxenon compound Xe(CF
3)
2 can be made by irradiating hexafluoroethane to generate CF
3· radicals and passing the gas over XeF
2. The resulting waxy white solid decomposes completely within 4 hours at room temperature.[16]
The XeF+ cation is formed by combining xenon difluoride with a strong fluoride acceptor, such as an excess of liquid antimony pentafluoride (SbF
5):
- XeF
2 + SbF
5 → XeF+
+ SbF−
6
Adding xenon gas to this pale yellow solution at a pressure of 2-3 atm produces a green solution containing the paramagnetic Xe+
2 ion,[17] which contains a Xe−Xe bond: ("apf" denotes solution in liquid SbF
5)
- 3 Xe (g) + XeF+
(apf) + SbF
5 (l) ⇌ 2 Xe+
2 (apf) + SbF−
6 (apf)
This reaction is reversible; removing xenon gas from the solution causes the Xe+
2 ion to revert to xenon gas and XeF+
, and the color of the solution returns to a pale yellow.[18]
In the presence of liquid HF, dark green crystals can be precipitated from the green solution at −30 °C:
- Xe+
2 (apf) + 4 SbF−
6 (apf) → Xe+
2Sb
4F−
21 (s) + 3 F−
(apf)
X-ray crystallography indicates that the Xe-Xe bond length in this compound is 309 pm, indicating a very weak bond.[16] The Xe+
2 ion is isoelectronic with the I−
2 ion, which is also dark green.[19][20]
Coordination chemistry
Bonding in the XeF2 molecule is adequately described by Three-center four-electron bond model.
XeF2 can act as a ligand in coordination complexes of metals.[2] For example, in HF solution:
- Mg(AsF6)2 + 4 XeF2 → [Mg(XeF2)4](AsF6)2
Crystallographic analysis shows that the magnesium atom is coordinated to 6 fluorine atoms. Four of the fluorines are attributed to the four xenon difluoride ligands while the other two are a pair of cis-AsF−
6 ligands.[21]
A similar reaction is:
- Mg(AsF6)2 + 2 XeF2 → [Mg(XeF2)2](AsF6)2
In the crystal structure of this product the magnesium atom is octahedrally-coordinated and the XeF2 ligands are axial while the AsF−
6 ligands are equatorial.
Many such reactions with products of the form [Mx(XeF2)n](AF6)x have been observed, where M can be Ca, Sr, Ba, Pb, Ag, La, or Nd and A can be As, Sb or P.
Recently, a compound was synthesised where a metal atom was coordinated solely by XeF2 fluorine atoms:[22]
- 2 Ca(AsF6 )2 + 9 XeF2 → Ca2(XeF2)9(AsF6)4.
This reaction requires a large excess of xenon difluoride. The structure of the salt is such that half of the Ca2+ ions are coordinated by fluorine atoms from xenon difluoride, while the other Ca2+ ions are coordinated by both XeF2 and AsF−
6.
Applications
As a fluorinating agent
Xenon difluoride is a strong fluorinating and oxidising agent.[23][24] With fluoride ion acceptors, it forms XeF+
and Xe
2F+
3 species which are even more powerful fluorinators.[2]
Among the fluorination reactions that xenon difluoride undergoes are:
- Oxidative fluorination:
- Ph3TeF + XeF2 → Ph3TeF3 + Xe
- Reductive fluorination:
- 2 CrO2F2 + XeF2 → 2 CrOF3 + Xe +O2
- Aromatic fluorination:
- Alkene fluorination:
- Radical fluorination in radical decarboxylative fluorination reactions,[25] in Hunsdiecker-type reactions where xenon difluoride is used to generate the radical intermediate as well as the fluorine transfer source,[26] and in generating aryl radicals from aryl silanes:[27]
XeF
2 is selective about which atom it fluorinates, making it a useful reagent for fluorinating heteroatoms without touching other substituents in organic compounds. For example, it fluorinates the arsenic atom in trimethylarsine, but leaves the methyl groups untouched:[28]
- (CH
3)
3As + XeF
2 → (CH
3)
3AsF
2 + Xe
XeF
2 will also oxidatively decarboxylate carboxylic acids to the corresponding fluoroalkanes:[29][30]
- RCOOH + XeF2 → RF + CO2 + Xe + HF
Silicon tetrafluoride has been found to act as a catalyst in fluorination by XeF
2.[31]
As an etchant
Xenon difluoride is also used as an isotropic gaseous etchant for silicon, particularly in the production of microelectromechanical systems, (MEMS), as first demonstrated in 1995.[32] Commercial systems use pulse etching with an expansion chamber [33] Brazzle, Dokmeci, et al., describe this process:[34]
The mechanism of the etch is as follows. First, the XeF2 adsorbs and dissociates to xenon (Xe) and fluorine (F) on the surface of silicon. Fluorine is the main etchant in the silicon etching process. The reaction describing the silicon with XeF2 is
- 2 XeF2 + Si → 2 Xe + SiF4
XeF2 has a relatively high etch rate and does not require ion bombardment or external energy sources in order to etch silicon.
References
- ↑ Hindermann, D. K., Falconer, W. E. (1969). "Magnetic Shielding of 19F in XeF2". J. Chem. Phys. 50 (3): 1203. Bibcode:1969JChPh..50.1203H. doi:10.1063/1.1671178.
- 1 2 3 4 5 Melita Tramšek; Boris Žemva (2006). "Synthesis, Properties and Chemistry of Xenon(II) Fluoride" (PDF). Acta Chim. Slov. 53 (2): 105–116. doi:10.1002/chin.200721209.
- 1 2 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ "MSDS: xenon difluoride" (PDF). BOC Gases. Retrieved 2010-06-01.
- ↑ James L. Weeks; Max S. Matheson. "Xenon Difluoride". Inorg. Synth. 8. doi:10.1002/9780470132395.ch69.
- ↑ Kim, M.; Debessai, M.; Yoo, C. S. (2010). "Two- and three-dimensional extended solids and metallization of compressed XeF2". Nature Chemistry. 2 (9): 784–788. Bibcode:2010NatCh...2..784K. doi:10.1038/nchem.724. PMID 20729901.
- ↑ Kurzydłowski, D.; Zaleski-Ejgierd, P.; Grochala, W.; Hoffmann, R. (2011). "Freezing in Resonance Structures for Better Packing: XeF2Becomes (XeF+)(F−) at Large Compression". Inorganic Chemistry. 50 (8): 3832–3840. doi:10.1021/ic200371a. PMID 21438503.
- ↑ Tius, M. A. (1995). "Xenon difluoride in synthesis". Tetrahedron. 51 (24): 6605–6634. doi:10.1016/0040-4020(95)00362-C.
- ↑ Chernick, CL and Claassen, HH and Fields, PR and Hyman, HH and Malm, JG and Manning, WM and Matheson, MS and Quarterman, LA and Schreiner, F. and Selig, HH; et al. (1962). "Fluorine Compounds of Xenon and Radon". Science. 138 (3537): 136–138. Bibcode:1962Sci...138..136C. doi:10.1126/science.138.3537.136. PMID 17818399.
- ↑ Hoppe, R.; Daehne, W.; Mattauch, H.; Roedder, K. (1962). "Fluorination of Xenon". Angew. Chem. Int. Ed. Engl. 1 (11): 599. doi:10.1002/anie.196205992.
- ↑ Hoppe, R. (1964). "Die Valenzverbindungen der Edelgase". Angewandte Chemie. 11: 455. doi:10.1002/ange.19640761103. First review on the subject by the pioneer of covalent noble gas compounds.
- ↑ Weeks, J.; Matheson, M.; Chernick, C., (1962). "Photochemical Preparation of Xenon Difluoride" Photochemical Preparation of Xenon Difluoride". J. Am. Chem. Soc. 84 (23): 4612–4613. doi:10.1021/ja00882a063.
- ↑ Williamson, Stanley M.; Sladky, Friedrich O.; Bartlett, Neil (1968). "Xenon Difluoride". Inorg. Synth. 11: 147–151. doi:10.1002/9780470132425.ch31.
- ↑ Šmalc, Andrej; Lutar, Karel; Kinkead, Scott A. (1992). "Xenon Difluoride (Modification)". Inorg. Synth. 29: 1–4. doi:10.1002/9780470132609.ch1.
- ↑ Morrow, S. I.; Young, A. R. (1965). "The Reaction of Xenon with Dioxygen Difluoride. A New Method for the Synthesis of Xenon Difluoride". Inorganic Chemistry. 4 (5): 759–760. doi:10.1021/ic50027a038.
- 1 2 Harding, Charlie; Johnson, David Arthur; Janes, Rob (2002). Elements of the p block. Contributor Charlie Harding, David Arthur Johnson, Rob Janes. Royal Society of Chemistry (Great Britain), Open University. ISBN 0-85404-690-9.
- ↑ Brown, D. R.; Clegg, M. J.; Downs, A. J.; Fowler, R. C.; Minihan, A. R.; Norris, J. R.; Stein, L. . (1992). "The dixenon(1+) cation: formation in the condensed phases and characterization by ESR, UV-visible, and Raman spectroscopy". Inorganic Chemistry. 31 (24): 5041–5052. doi:10.1021/ic00050a023.
- ↑ Stein, L. .; Henderson, W. W. (1980). "Production of dixenon cation by reversible oxidation of xenon". Journal of the American Chemical Society. 102 (8): 2856–2857. doi:10.1021/ja00528a065.
- ↑ Mackay, Kenneth Malcolm; Mackay, Rosemary Ann; Henderson, W. (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. ISBN 0-7487-6420-8.
- ↑ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 422. ISBN 0-12-352651-5.
- ↑ Tramšek, M.; Benkič, P.; Žemva, B. (2004). "First Compounds of Magnesium with XeF2". Inorg. Chem. 43 (2): 699–703. doi:10.1021/ic034826o.
- ↑ Tramšek, M.; Benkič, P.; Žemva, B. (2004). "The First Compound Containing a Metal Center in a Homoleptic Environment of XeF2 Molecules". Angewandte Chemie International Edition. 43 (26): 3456. doi:10.1002/anie.200453802.
- ↑ D. F. Halpem, "Xenon(II) Fluoride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York.
- ↑ Taylor, S.; Kotoris, C.; Hum, G., (1999). "Recent Advances in Electrophilic Fluorination". Tetrahedron. 55 (43): 12431–12477. doi:10.1016/S0040-4020(99)00748-6. A review of fluorination in general.
- ↑ Tius, M. A. (1995). "Xenon difluoride in synthesis". Tetrahedron. 51 (24): 6605–6634. doi:10.1016/0040-4020(95)00362-C.
- ↑ Patrick, T. B.; Darling, D. L. (1986). "Fluorination of activated aromatic systems with cesium fluoroxysulfate". J. Org. Chem. 51 (16): 3242–3244. doi:10.1021/jo00366a044.
- ↑ Lothian, A. P.; Ramsden, C. A. (1993). "Rapid fluorodesilylation of aryltrimethylsilanes using xenon difuoride: An efficient new route to aromatic fluorides". Synlett. 1993 (10): 753–755. doi:10.1055/s-1993-22596.
- ↑ W. Henderson (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. p. 150. ISBN 0-85404-617-8.
- ↑ Patrick, Timothy B.; Johri, Kamalesh K.; White, David H.; Bertrand, William S.; Mokhtar, Rodziah; Kilbourn, Michael R.; Welch, Michael J. (1986). "Replacement of the carboxylic acid function with fluorine". Can. J. Chem. 64: 138. doi:10.1139/v86-024.
- ↑ Grakauskas, Vytautas (1969). "Aqueous fluorination of carboxylic acid salts". J. Org. Chem. 34 (8): 2446. doi:10.1021/jo01260a040.
- ↑ Tamura Masanori; Takagi Toshiyuki; Shibakami Motonari; Quan Heng-Dao; Sekiya Akira (1998). "Fluorination of olefins with xenon difluoride-silicon tetrafluoride". Fusso Kagaku Toronkai Koen Yoshishu (in Japanese). Japan. 22: 62–63. Journal code: F0135B; accession code: 99A0711841.
- ↑ Chang, F.; Yeh, R.; Lin, G.; Chu, P.;Hoffman, E.; Kruglick, E.; Pister, K.; Hecht, M; "Gas-phase silicon micromachining with xenon difluoride", SPIE Proc. V2641, 1995, pages 117-128.
- ↑ Chu, P.; Chen, J.; Chu, P.; Lin, G.; Huang, J.; Warneke, B; Pister, K.; "Controlled Pulse-Etching with Xenon Difluoride", Int. Conf. Solid State Sensors and Actuators, 1997 (Transducers 97), pages 665-668.
- ↑ Brazzle, J.D.; Dokmeci, M.R.; Mastrangelo, C.H.; Modeling and characterization of sacrificial polysilicon etching using vapor-phase xenon difluoride, 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), 2004, pages 737-740.
Additional reading
- Greenwood, Norman Neill; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 894. ISBN 0-7506-3365-4.




