Iron(II) bromide
 | |
| Names | |
|---|---|
| IUPAC name
Iron(II) bromide | |
| Other names
Ferrous bromide | |
| Identifiers | |
| 7789-46-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 74218 |
| ECHA InfoCard | 100.029.244 |
| PubChem | 659170 |
| |
| |
| Properties | |
| FeBr2 | |
| Molar mass | 215.65 g mol−1 |
| Appearance | yellow-brown solid |
| Density | 4.63 g cm−3, solid |
| Melting point | 684 °C (1,263 °F; 957 K) (anhydrous) 27 °C (Hexahydrate) |
| Boiling point | 934 °C (1,713 °F; 1,207 K) |
| soluble | |
| Solubility in other solvents | THF, methanol, ethanol |
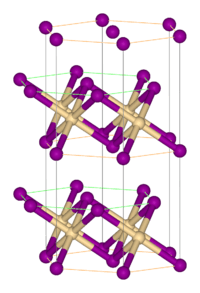
| Structure | |
| Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| Main hazards | none |
| R-phrases | R20 R36/37/38 |
| S-phrases | S26 S36 |
| Related compounds | |
| Other anions |
Iron(II) chloride |
| Other cations |
iron(III) bromide |
| Related compounds |
VBr2 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Iron(II) bromide is the chemical compound with the chemical formula FeBr2. The anhydrous compound is a yellow or brownish-colored paramagnetic solid. It is a common precursor to other iron compounds in research laboratory. Several hydrates of FeBr2 are also known.
Structure
Like most metal halides, FeBr2 adopts a polymeric structure consisting of isolated metal centers cross-linked with halides. It crystallizes with the CdI2 structure, featuring close-packed layers of bromide ions, between which are located Fe(II) ions in octahedral holes.[1] The packing of the halides is slightly different from that for FeCl2, which adopts the CdCl2 motif.
Synthesis and reactions
FeBr2 is synthesized using a methanol solution of concentrated hydrobromic acid and iron powder. gives the methanol solvate [Fe(MeOH)6]Br2 together with hydrogen gas. Heating the methanol complex in a vacuum gives pure FeBr2.[2] Iron(II) bromide cannot be formed by the reaction of iron and bromine, because that reaction would produce ferric bromide.
FeBr2 reacts with 2 equivalents of (C2H5)4NBr to give [(C2H5)4N]2FeBr4.[3]
FeBr2 reacts with bromide and bromine to form the intensely colored, mixed-valence species [FeBr3Br9]−.[4]
FeBr2 is a weak reducing agent, as are all ferrous compounds.
References
- ↑ Haberecht, J.; Borrmann, H.; Kniep, R. "Refinement of the Crystal Structure of Iron Dibromide, FeBr2" Zeitschrift für Kristallographie - New Crystal Structures 2001, vol. 216, p. 510. doi:10.1524/ncrs.2001.216.14.544
- ↑ G. Winter, "Iron(II) Halides" "Inorganic Syntheses" 1973, volume 14, pages 101-104. doi: 10.1002/9780470132456.ch20
- ↑ N. S. Gill, F.. B. Taylor Inorganic Syntheses 1967, volume 9, page 136-142. doi: 10.1002/9780470132401.ch37
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5