Chromium pentafluoride
 | |
| Names | |
|---|---|
| IUPAC name
Chromium(V) fluoride | |
| Other names
Chromium fluoride, Chromium(V) fluoride, Pentafluorochromium, Pentafluoridochromium | |
| Identifiers | |
| 14884-42-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4574207 |
| PubChem | 5460742 |
| |
| |
| Properties | |
| CrF5 | |
| Molar mass | 291.71 g/mol |
| Appearance | red crystals[1] |
| Density | 2.89 g/cm3[1] |
| Melting point | 34 °C (93 °F; 307 K)[1] |
| Boiling point | 117 °C (243 °F; 390 K)[1] |
| Structure | |
| Orthorhombic[1] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5.[2] It is a red volatile solid that melts at 30 °C, which easily hydrolyses to chromium(III) and chromium(VI).[3] It has the same crystal structure as vanadium pentafluoride.[4] It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.[5]
Chromium pentafluoride is one of the products of the action of fluorine on a mixture of potassium and chromic chlorides.[6]
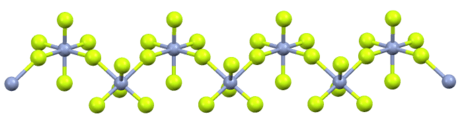
In terms of its structure, the compound is a one-dimensional coordination polymer. Each Cr(V) center has octahedral molecular geometry.[7]
See also
References
- 1 2 3 4 5 Perry, Dale L. (2011). Handbook of Inorganic Compounds, Second Edition. Boca Raton, Florida: CRC Press. p. 125. ISBN 978-1-43981462-8. Retrieved 2014-01-10.
- ↑ Jacques Guertin; James A. Jacobs; Cynthia P. Avakian, eds. (2004). Chromium(VI) Handbook. CRC Press. p. 30. ISBN 9780203487969.
- ↑ Amit Aora (2005). Text Book Of Inorganic Chemistry. Discovery Publishing House. p. 649.
- ↑ A. G. Sharpe (1983). Advances in Inorganic Chemistry. 27. Academic Press. p. 103. ISBN 9780080578767.
- ↑ Riedel, Sebastian; Kaupp, Martin (2009). "The highest oxidation states of the transition metal elements" (PDF). Coordination Chemistry Reviews. 253 (5–6): 606–624. doi:10.1016/j.ccr.2008.07.014.
- ↑ A. G. Sharpe (December 2012). J.H. Simons, ed. Fluorine Chemistry. 2. Elsevier. p. 24. ISBN 9780323145435.
- ↑ "The structures of CrF5 and CrF5*SbF5" Shorafa, H.; Seppelt, K. Zeitschrift für Anorganische und Allgemeine Chemie 2009, vol. 635, p112-p114.
This article is issued from Wikipedia - version of the 10/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.