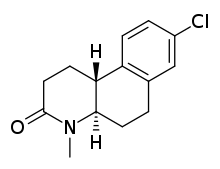
Bexlosteride
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | LY 300502 |
| CAS Number |
148905-78-6 |
| PubChem (CID) | 166562 |
| ChemSpider |
145762 |
| UNII |
36X732P4P0 |
| ChEMBL |
CHEMBL24955 |
| Chemical and physical data | |
| Formula | C14H16ClNO |
| Molar mass | 249.73594 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Bexlosteride is a potent and noncompetitive inhibitor of the enzyme 5α-reductase related to finasteride and dutasteride.[1][2] It is selective for the type I isoform of the enzyme.[1] It advanced to Phase III clinical trials, but development was halted at that stage, and it was never marketed.[3][4]
See also
References
- 1 2 Chang, Chawnshang (2002). Androgens and androgen receptor : mechanisms, functions, and clinical application. Boston: Kluwer Academic Publishers. ISBN 1-4020-7188-4.
- ↑ Lednicer, Daniel (2008). Strategies for Organic Drug Synthesis and Design. New York: Wiley-Interscience. ISBN 0-470-19039-6.
- ↑ "Drug Profile: Bexlosteride". Adis Insight.
- ↑ Reaxys entry for bexlosteride: Reaxys Registry Number: 6635310
This article is issued from Wikipedia - version of the 12/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.