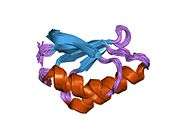
ATOX1
| View/Edit Human | View/Edit Mouse |
ATOX1 is a copper metallochaperone protein that is encoded by the ATOX1 gene in humans.[3][4] In mammals, ATOX1 plays a key role in copper homeostasis as it delivers copper from the cytosol to transporters ATP7A and ATP7B.[5][6][7] Homologous proteins are found in a wide variety of eukaryotes, including Saccharomyces cerevisiae as ATX1, and all contain a conserved metal binding domain.[5][8]
Function
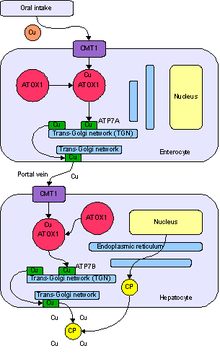
ATOX1 is an abbreviation of the full name Antioxidant Protein 1. The nomenclature stems from initial characterization that showed that ATOX1 protected cells from reactive oxygen species. Since then, the primary role of ATOX1 has been established as a copper metallochaperone protein found in the cytoplasm of eukaryotes.[5] A metallochaperone is an important protein that has metal trafficking and sequestration roles. As a metal sequestration protein, ATOX1 is capable of binding free metals in vivo, in order to protect cells from generation of reactive oxygen species and mismetallation of metalloproteins. As a metal trafficking protein, ATOX1 is responsible for shuttling copper from the cytosol to ATPase transporters ATP7A and ATP7B that move copper to the trans-Golgi network or secretory vesicles.[5][6][7] In Saccharomyces cerevisiae, ATX1 delivers Cu(I) to a homologous transporter, Ccc2. The delivery of copper to ATPase transporters is vital for the subsequent insertion of copper into ceruloplasmin, a ferroxidase required for iron metabolism, within the golgi apparatus.[5] In addition to the metallochaperone function, recent reports have characterized ATOX1 as a cyclin D1 transcription factor.[6]
Structure & metal coordination
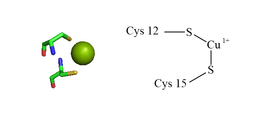
ATOX1 has a ferrodoxin-like βαββαβ fold and coordinates to Cu(I) via a MXCXXC binding motif located in between the first β-sheet and α-helix.[5][7] The metal binding motif is largely solvent exposed in Apo-ATOX1 and a conformational change is induced upon coordination to Cu(I).[7][8] Cu(I) is coordinated in a distorted linear geometry to sulfurs of cystine to form a bond angle of 120°.[7] The overall -1 charge of the primary coordination sphere is stabilized through the secondary coordination sphere that contains a proximal positively charged lysine.[7][8] ATOX1 also binds Hg(II), Cd(II), Ag(I), and cisplatin via this motif, but a physiological role, if any, is not yet known.[7]
Metal transfer

ATOX1 transfers Cu(I) to transporters ATP7A and ATP7B.[5][6][7] Transfer occurs via a ligand exchange mechanism, where Cu(I) transiently adopts a 3-coordinate geometry with cysteine ligands from ATOX1 and the associated transporter.[7] The ligand exchange mechanism allows for faster exchange than a diffusion mechanism and imparts specificity for both the metal and transporter.[9] Since the ligand exchange accelerates that transfer and the reaction has a shallow thermodynamic gradient, it is said to be under kinetic control rather than thermodynamic control.[7][9]
Clinical significance
Although there are presently no known diseases directly associated with ATOX1 malfunction, there is currently active research in a few areas:
- There is a link between ATOX1 levels and sensitivity of cells for Pt-based drugs like cisplatin.[7]
- The mechanism of ammonium tetrathiomolybdate [NH4]2MoS4 treatment of Wilson's Disease is under review. Since ATOX1 forms a stable complex tetrathiomolybdate, it is being studied as the potential therapeutic target.[10][11]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD (May 1997). "Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis". J Biol Chem. 272 (14): 9221–6. doi:10.1074/jbc.272.14.9221. PMID 9083055.
- ↑ "Entrez Gene: ATOX1 ATX1 antioxidant protein 1 homolog (yeast)".
- 1 2 3 4 5 6 7 Bertini I, Gray HB, Steifel EI, Valentine JS (2006). Biological Inorganic Chemistry, Structure and Reactivity. University Science Books. ISBN 1891389432.
- 1 2 3 4 Banci L (2013). Metallomics and the cell. Dordrecht: Springer. ISBN 978-94-007-5561-1.
- 1 2 3 4 5 6 7 8 9 10 11 Maret W, Wedd A (2014). Binding, transport and storage of metal ions in biological cells. [S.l.]: Royal Soc Of Chemistry. ISBN 978-1-84973-599-5.
- 1 2 3 Boal AK, Rosenzweig AC (Oct 2009). "Structural biology of copper trafficking". Chemical Reviews. 109 (10): 4760–4779. doi:10.1021/cr900104z. PMID 19824702.
- 1 2 Robinson NJ, Winge DR (7 June 2010). "Copper metallochaperones". Annual Review of Biochemistry. 79 (1): 537–562. doi:10.1146/annurev-biochem-030409-143539. PMID 20205585.
- ↑ Alvarez HM, Xue Y, Robinson CD, Canalizo-Hernández MA, Marvin RG, Kelly RA, Mondragón A, Penner-Hahn JE, O'Halloran TV (Jan 2010). "Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation". Science. 327 (5963): 331–334. doi:10.1126/science.1179907. PMID 19965379.
- ↑ Mjos KD, Orvig C (Apr 2014). "Metallodrugs in medicinal inorganic chemistry". Chemical Reviews. 114 (8): 4540–4563. doi:10.1021/cr400460s. PMID 24456146.
Further reading
- Hung IH, Casareno RL, Labesse G, Mathews FS, Gitlin JD (1998). "HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense.". J. Biol. Chem. 273 (3): 1749–54. doi:10.1074/jbc.273.3.1749. PMID 9430722.
- Larin D, Mekios C, Das K, Ross B, Yang AS, Gilliam TC (1999). "Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p.". J. Biol. Chem. 274 (40): 28497–504. doi:10.1074/jbc.274.40.28497. PMID 10497213.
- Hamza I, Schaefer M, Klomp LW, Gitlin JD (1999). "Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis.". Proc. Natl. Acad. Sci. U.S.A. 96 (23): 13363–8. doi:10.1073/pnas.96.23.13363. PMC 23953
 . PMID 10557326.
. PMID 10557326. - Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC (2000). "Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins.". Nat. Struct. Biol. 7 (9): 766–71. doi:10.1038/78999. PMID 10966647.
- Boultwood J, Strickson AJ, Jabs EW, Cheng JF, Fidler C, Wainscoat JS (2000). "Physical mapping of the human ATX1 homologue (HAH1) to the critical region of the 5q- syndrome within 5q32, and immediately adjacent to the SPARC gene.". Hum. Genet. 106 (1): 127–9. doi:10.1007/s004390051020. PMID 10982193.
- Walker JM, Tsivkovskii R, Lutsenko S (2002). "Metallochaperone Atox1 transfers copper to the NH2-terminal domain of the Wilson's disease protein and regulates its catalytic activity.". J. Biol. Chem. 277 (31): 27953–9. doi:10.1074/jbc.M203845200. PMID 12029094.
- Moore SD, Helmle KE, Prat LM, Cox DW (2003). "Tissue localization of the copper chaperone ATOX1 and its potential role in disease.". Mamm. Genome. 13 (10): 563–8. doi:10.1007/s00335-002-2172-9. PMID 12420134.
- Liu PC, Koeller DM, Kaler SG (2004). "Genomic organization of ATOX1, a human copper chaperone.". BMC Genet. 4: 4. doi:10.1186/1471-2156-4-4. PMC 150598
 . PMID 12594858.
. PMID 12594858. - Strausak D, Howie MK, Firth SD, Schlicksupp A, Pipkorn R, Multhaup G, Mercer JF (2003). "Kinetic analysis of the interaction of the copper chaperone Atox1 with the metal binding sites of the Menkes protein.". J. Biol. Chem. 278 (23): 20821–7. doi:10.1074/jbc.M212437200. PMID 12679332.
- Ralle M, Lutsenko S, Blackburn NJ (2003). "X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines.". J. Biol. Chem. 278 (25): 23163–70. doi:10.1074/jbc.M303474200. PMID 12686548.
- Lutsenko S, Tsivkovskii R, Walker JM (2003). "Functional properties of the human copper-transporting ATPase ATP7B (the Wilson's disease protein) and regulation by metallochaperone Atox1.". Ann. N. Y. Acad. Sci. 986: 204–11. doi:10.1111/j.1749-6632.2003.tb07161.x. PMID 12763797.
- Wernimont AK, Yatsunyk LA, Rosenzweig AC (2004). "Binding of copper(I) by the Wilson disease protein and its copper chaperone.". J. Biol. Chem. 279 (13): 12269–76. doi:10.1074/jbc.M311213200. PMID 14709553.
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW (2005). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation.". Nat. Biotechnol. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197.
- Anastassopoulou I, Banci L, Bertini I, Cantini F, Katsari E, Rosato A (2004). "Solution structure of the apo and copper(I)-loaded human metallochaperone HAH1.". Biochemistry. 43 (41): 13046–53. doi:10.1021/bi0487591. PMID 15476398.
- Banci L, Bertini I, Ciofi-Baffoni S, Chasapis CT, Hadjiliadis N, Rosato A (2005). "An NMR study of the interaction between the human copper(I) chaperone and the second and fifth metal-binding domains of the Menkes protein.". FEBS J. 272 (3): 865–71. doi:10.1111/j.1742-4658.2004.04526.x. PMID 15670166.
- Jeney V, Itoh S, Wendt M, Gradek Q, Ushio-Fukai M, Harrison DG, Fukai T (2005). "Role of antioxidant-1 in extracellular superoxide dismutase function and expression.". Circ. Res. 96 (7): 723–9. doi:10.1161/01.RES.0000162001.57896.66. PMID 15761197.