Aldehyde dehydrogenase 3 family, member A1
| View/Edit Human | View/Edit Mouse |
Aldehyde dehydrogenase, dimeric NADP-preferring is an enzyme that in humans is encoded by the ALDH3A1 gene.[3][4][5]
Aldehyde dehydrogenases oxidize various aldehydes to the corresponding acids. They are involved in the detoxification of alcohol-derived acetaldehyde and in the metabolism of corticosteroids, biogenic amines, neurotransmitters, and lipid peroxidation. The enzyme encoded by this gene forms a cytoplasmic homodimer that preferentially oxidizes aromatic aldehyde substrates. The gene is located within the Smith-Magenis syndrome region on chromosome 17.[5]
ALDH3A1 expression is notably high in the cornea of mammalian species, comprising from 5 to 50% of soluble protein content, but is almost absent from the cornea of other vertebrates.[6]
Structure and mechanism
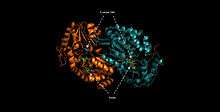
ALDH3A1 is a homodimer consisting of alpha helices (43.8%), beta sheets (4.2%), p-loop turns (28.2%) and random coils (23.8%).[7] The catalytic residue–Cys244—is located on an active site that contains a Rossman fold that binds the enzyme's cofactor, NAD(P)+.[8]
ALDH3A1’s catalytic mechanism mirrors that of other enzymes of the aldehyde dehydrogenase family. The sulfur atom of Cys244 attacks the carbonyl of the aldehyde substrate in a nucleophilic attack that releases a hydride ion. The hydride ion is accepted by the NAD(P)+ bound to the Rossman fold. Unique interactions between the cofactor and the Rossman fold facilitate an isomerization of the enzyme that releases the cofactor while maintaining the integrity of the active site.[9] A water molecule enters the active site and is subsequently activated by a glutamate residue. The activated water then attacks the thioester enzyme-substrate complex in nucleophilic reaction that regenerates the free enzyme, and releases the corresponding carboxylic acid.
Involvement in lipid peroxidation
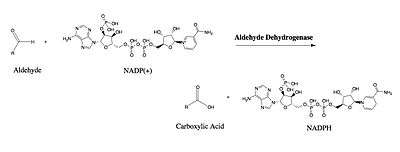
Electronic excitations of alkene and aromatic functional groups allow certain nucleic acids, proteins, fatty acids and organic molecules to absorb ultraviolet radiation (UVR). Moderate UVR exposure oxidizes specific proteins that eventually serve as signaling agents for an array of metabolic and inflammatory pathways.[7] Overexposure to UVR, on the other hand, can be detrimental to the tissue. In the presence of molecular oxygen, UVR leads to the formation of reactive oxygen species (ROS) that are implicated in many degradation pathways.[10] In the case of lipid peroxidation, ROS react with polyunsaturated fatty acids situated in the lipid bilayer of the cell membrane to produce lipid radicals. These lipid radicals propagate, further damaging the lipid bilayer and producing lipid hydroperoxides. The eventual degradation of lipid hydroperoxides releases a wide variety of aldehydes, which, owing to their stability and ability to react cellular nucleophiles,[10] are both cytotoxic and genotoxic in nature. ALDH3A1 plays a critical role in the metabolism of these aldehydes to their corresponding carboxylic acids in mammalian cornea and saliva. 4-Hydroxynonenal (4HNE)—which ALDH3A1 metabolizes with Vmax of 27,754 moles NADPH/min•mg and an apparent Km of 362 micromolar[7] —is the most abundant aldehyde produced in the LPO of arachidonic acid and linoleic acid.[11][12] Its stability and multiple sites of reactivity (carbon-carbon double bond, hydroxyl group, and carbonyl) make 4HNE a potent inhibitor of cellular growth, enzyme activities, calcium sequestration, and protein synthesis. It is also involved in the consumption of glutathione and the alteration of signal transduction and gene expression.[13][14][15][16][17]
Role in the cornea
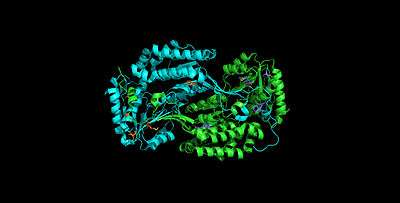
ALDH3A1 comprises approximately 10-40% of the water-soluble protein in the mammalian cornea.[18][19] Direct exposure to UVR and molecular oxygen, make the cornea susceptible to ROS and 4HNE. Studies in which rabbits were transfected with genes that allow them to overexpress human ALDH3A1 in their corneal stromal fibroblasts document ALDH3A1's most critical function is to protect the cornea from oxidative stresses. In the cornea ALDH3A1: (1) prevents the formation of 4-HNE protein adducts that would impeded proteins’ function; (2) is more effective at metabolizing 4-HNE than other comparable agents such as glutathione (GSH); (3) protects the corneal cells from 4-HNE induced apoptosis; (4) reduces consumption of GSH by relieving 4HNE GSH adducts; (5) and relieves 4-HNE’s inhibition of the 20S protease activity.[20]
Suicide response to UVR
However, only a fraction of the total concentration of ALDH3A1 in the cornea is used for metabolizing aldehydes. This observation has sparked multiple investigations of ALDH3A1’s role beyond aldehyde metabolism.[21] Although the full scope of ALDH3A1’s function is yet to be firmly established, there is strong evidence suggesting that ALDH3A1 serves to maintain the cellular redox balance as well as the structural integrity and transparency of the cornea. One study elucidates that ALDH3A1 not only indirectly protects the cornea from UVR induced oxidative stress by metabolizing aldehydes, but also protects the tissue directly, by competitively absorbing UVR in a “suicide response”[6] that reduces damage to other proteins of the cornea[7] In fact, 50% percent of the UVR that the cornea is exposed to is absorbed by ADLH3A1. ALDH3A1’s absorption of UVR oxidizes several key amino acid residues, leading to conformational changes that convert the alpha and beta sheets into random coils. These conformational changes ultimately relieve the dimer structure. This loss of secondary and tertiary structure leads to protein aggregation and complete loss of enzymatic activity.[7] Peptide mapping and spectroscopic experiments reveal that the loss of activity is not a result of Cys244 oxidation (which, together with the active site, remains intact during photo-excitation), but instead, due to the degradation of other key amino residues (most notably methionine and tryptophan). These amino acid residues degrade under oxidative stress, leading to the formation of non-reducible cross-links that stabilize the soluble aggregates.[7] Tryptophan for instance is doubly oxidized to generate ROSs such as H2O2, which elicit further oxidation and adduction.[22] Nevertheless, the abundance of ALDH3A1 in the cornea ensures that this suicide response neither impedes with aldehyde metabolism nor leads to the formation of insoluble aggregates that would affect the transparency of the cornea.[23]

Consequences of ALDH3A1 deficiency
Further clarification of ALDH3A1’s role in the cornea has been provided by gene-knockout studies in which genes encoding ALDH3A1 were removed from the mice genome. It was found that ALDH3A1-null mice exhibited lower proteasome activity, higher rates of protein degradation/oxidation, and higher GSH, 4HNE and malondialdehyde protein adduct levels—all of which contributed to the development of cataracts and opacities in the subcapular regions of the cornea within one month of age.[21] These observations on ALDH3A1-null mice reaffirm that ALDH3A1’s role extends beyond enzymatic metabolism; encompassing functions in maintenance of the structural integrity and transparency of the cornea.
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Hiraoka LR, Hsu L, Hsieh CL (Jul 1995). "Assignment of ALDH3 to human chromosome 17p11.2 and ALDH5 to human chromosome 9p13". Genomics. 25 (1): 323–5. doi:10.1016/0888-7543(95)80150-K. PMID 7774944.
- ↑ Hsu LC, Chang WC, Shibuya A, Yoshida A (Mar 1992). "Human stomach aldehyde dehydrogenase cDNA and genomic cloning, primary structure, and expression in Escherichia coli". J Biol Chem. 267 (5): 3030–7. PMID 1737758.
- 1 2 "Entrez Gene: ALDH3A1 aldehyde dehydrogenase 3 family, memberA1".
- 1 2 Estey T, Piatigorsky J, Lassen N, Vasiliou V (January 2007). "ALDH3A1: a corneal crystallin with diverse functions". Exp. Eye Res. 84 (1): 3–12. doi:10.1016/j.exer.2006.04.010. PMID 16797007.
- 1 2 3 4 5 6 Estey T, Chen Y, Carpenter JF, Vasiliou V (2010). "Structural and functional modifications of corneal crystallin ALDH3A1 by UVB light". PLoS ONE. 5 (12): e15218. doi:10.1371/journal.pone.0015218. PMC 3006428
 . PMID 21203538.
. PMID 21203538. - ↑ Liu ZJ, Sun YJ, Rose J, Chung YJ, Hsiao CD, Chang WR, Kuo I, Perozich J, Lindahl R, Hempel J, Wang BC (April 1997). "The first structure of an aldehyde dehydrogenase reveals novel interactions between NAD and the Rossmann fold". Nat. Struct. Biol. 4 (4): 317–26. doi:10.1038/nsb0497-317. PMID 9095201.
- ↑ Perez-Miller SJ, Hurley TD (June 2003). "Coenzyme isomerization is integral to catalysis in aldehyde dehydrogenase". Biochemistry. 42 (23): 7100–9. doi:10.1021/bi034182w. PMID 12795606.
- 1 2 van Kuijk FJ (December 1991). "Effects of ultraviolet light on the eye: role of protective glasses". Environ. Health Perspect. 96: 177–84. doi:10.1289/ehp.9196177. PMC 1568237
 . PMID 1820264.
. PMID 1820264. - ↑ Benedetti A, Comporti M, Esterbauer H (November 1980). "Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids". Biochim. Biophys. Acta. 620 (2): 281–96. doi:10.1016/0005-2760(80)90209-x. PMID 6254573.
- ↑ Esterbauer H, Schaur RJ, Zollner H (1991). "Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes". Free Radic. Biol. Med. 11 (1): 81–128. doi:10.1016/0891-5849(91)90192-6. PMID 1937131.
- ↑ Dianzani MU (June 1998). "4-Hydroxynonenal and cell signalling". Free Radic. Res. 28 (6): 553–60. doi:10.3109/10715769809065811. PMID 9736307.
- ↑ Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU (December 1998). "HNE interacts directly with JNK isoforms in human hepatic stellate cells". J. Clin. Invest. 102 (11): 1942–50. doi:10.1172/JCI1413. PMC 509146
 . PMID 9835619.
. PMID 9835619. - ↑ Leonarduzzi G, Arkan MC, Başağa H, Chiarpotto E, Sevanian A, Poli G (May 2000). "Lipid oxidation products in cell signaling". Free Radic. Biol. Med. 28 (9): 1370–8. doi:10.1016/s0891-5849(00)00216-1. PMID 10924856.
- ↑ Kumagai T, Kawamoto Y, Nakamura Y, Hatayama I, Satoh K, Osawa T, Uchida K (July 2000). "4-hydroxy-2-nonenal, the end product of lipid peroxidation, is a specific inducer of cyclooxygenase-2 gene expression". Biochem. Biophys. Res. Commun. 273 (2): 437–41. doi:10.1006/bbrc.2000.2967. PMID 10873624.
- ↑ Feng Z, Hu W, Tang MS (June 2004). "Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis". Proc. Natl. Acad. Sci. U.S.A. 101 (23): 8598–602. doi:10.1073/pnas.0402794101. PMC 423240
 . PMID 15187227.
. PMID 15187227. - ↑ Pappa A, Sophos NA, Vasiliou V (January 2001). "Corneal and stomach expression of aldehyde dehydrogenases: from fish to mammals". Chem. Biol. Interact. 130-132 (1-3): 181–91. doi:10.1016/s0009-2797(00)00233-7. PMID 11306042.
- ↑ Piatigorsky J (November 2001). "Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the "refracton" hypothesis". Cornea. 20 (8): 853–8. doi:10.1097/00003226-200111000-00015. PMID 11685065.
- ↑ Black W, Chen Y, Matsumoto A, Thompson DC, Lassen N, Pappa A, Vasiliou V (May 2012). "Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal". Free Radic. Biol. Med. 52 (9): 1937–44. doi:10.1016/j.freeradbiomed.2012.02.050. PMC 3457646
 . PMID 22406320.
. PMID 22406320. - 1 2 Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, Duester G, Day BJ, Huang J, Hines LM, Vasiliou V (August 2007). "Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(-/-)/Aldh1a1(-/-) knock-out mice". J. Biol. Chem. 282 (35): 25668–76. doi:10.1074/jbc.M702076200. PMC 2253645
 . PMID 17567582.
. PMID 17567582. - ↑ Davies MJ (January 2004). "Reactive species formed on proteins exposed to singlet oxygen". Photochem. Photobiol. Sci. 3 (1): 17–25. doi:10.1039/b307576c. PMID 14743273.
- ↑ Piatigorsky J (April 1998). "Gene sharing in lens and cornea: facts and implications". Prog Retin Eye Res. 17 (2): 145–74. doi:10.1016/s1350-9462(97)00004-9. PMID 9695791.
Further reading
- Yoshida A (1993). "Molecular genetics of human aldehyde dehydrogenase.". Pharmacogenetics. 2 (4): 139–47. doi:10.1097/00008571-199208000-00001. PMID 1306115.
- Vasiliou V, Bairoch A, Tipton KF, Nebert DW (2000). "Eukaryotic aldehyde dehydrogenase (ALDH) genes: human polymorphisms, and recommended nomenclature based on divergent evolution and chromosomal mapping.". Pharmacogenetics. 9 (4): 421–34. PMID 10780262.
- Eckey R, Timmann R, Hempel J, et al. (1991). "Biochemical, immunological, and molecular characterization of a "high Km" aldehyde dehydrogenase.". Adv. Exp. Med. Biol. 284: 43–52. doi:10.1007/978-1-4684-5901-2_6. PMID 1905102.
- Yin SJ, Vagelopoulos N, Wang SL, Jörnvall H (1991). "Structural features of stomach aldehyde dehydrogenase distinguish dimeric aldehyde dehydrogenase as a 'variable' enzyme. 'Variable' and 'constant' enzymes within the alcohol and aldehyde dehydrogenase families.". FEBS Lett. 283 (1): 85–8. doi:10.1016/0014-5793(91)80559-L. PMID 2037078.
- Santisteban I, Povey S, West LF, et al. (1986). "Chromosome assignment, biochemical and immunological studies on a human aldehyde dehydrogenase, ALDH3.". Ann. Hum. Genet. 49 (Pt 2): 87–100. doi:10.1111/j.1469-1809.1985.tb01680.x. PMID 4073832.
- Teng YS (1981). "Stomach aldehyde dehydrogenase: report of a new locus.". Hum. Hered. 31 (2): 74–7. doi:10.1159/000153181. PMID 7228061.
- Dyck LE (1995). "Polymorphism of a class 3 aldehyde dehydrogenase present in human saliva and in hair roots.". Alcohol. Clin. Exp. Res. 19 (2): 420–6. doi:10.1111/j.1530-0277.1995.tb01525.x. PMID 7625577.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides.". Gene. 138 (1-2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Hsu LC, Yoshida A (1993). "Human stomach aldehyde dehydrogenase, ALDH3.". Adv. Exp. Med. Biol. 328: 141–52. doi:10.1007/978-1-4615-2904-0_16. PMID 8493892.
- Rogers GR, Markova NG, De Laurenzi V, et al. (1997). "Genomic organization and expression of the human fatty aldehyde dehydrogenase gene (FALDH).". Genomics. 39 (2): 127–35. doi:10.1006/geno.1996.4501. PMID 9027499.
- Tsukamoto N, Chang C, Yoshida A (1997). "Mutations associated with Sjögren-Larsson syndrome.". Ann. Hum. Genet. 61 (Pt 3): 235–42. doi:10.1046/j.1469-1809.1997.6130235.x. PMID 9250352.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library.". Gene. 200 (1-2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Rekha GK, Devaraj VR, Sreerama L, et al. (1998). "Inhibition of human class 3 aldehyde dehydrogenase, and sensitization of tumor cells that express significant amounts of this enzyme to oxazaphosphorines, by chlorpropamide analogues.". Biochem. Pharmacol. 55 (4): 465–74. doi:10.1016/S0006-2952(97)00475-9. PMID 9514081.
- Simpson JC, Wellenreuther R, Poustka A, et al. (2001). "Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing.". EMBO Rep. 1 (3): 287–92. doi:10.1093/embo-reports/kvd058. PMC 1083732
 . PMID 11256614.
. PMID 11256614. - Rodriguez-Zavala JS, Weiner H (2002). "Structural aspects of aldehyde dehydrogenase that influence dimer-tetramer formation.". Biochemistry. 41 (26): 8229–37. doi:10.1021/bi012081x. PMID 12081471.
- Yang M, Coles BF, Delongchamp R, et al. (2003). "Effects of the ADH3, CYP2E1, and GSTP1 genetic polymorphisms on their expressions in Caucasian lung tissue.". Lung Cancer. 38 (1): 15–21. doi:10.1016/S0169-5002(02)00150-2. PMID 12367788.