Tishchenko reaction
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide.[1] The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides. It is named after the Russian organic chemist Vyacheslav Evgen'evich Tishchenko (Вячеслав Евгеньевич Тищенко).[2]
In the related Cannizzaro reaction the base is sodium hydroxide and then the oxidation product is a carboxylic acid and the reduction product is an alcohol.
Examples
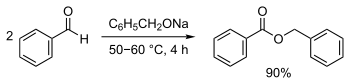
- Benzaldehyde reacts with sodium benzyloxide (generated from sodium and benzyl alcohol) to generate benzyl benzoate.[3]
- The Tishchenko reaction of acetaldehyde gives the commercially important solvent ethyl acetate; it is catalyzed by aluminium alcoholate.[4]
- The Tishchenko reaction is used to obtain isobutyl isobutyrate, a specialty solvent.[5]
- Hydroxypivalic acid neopentyl glycol ester is produced by a Tishchenko reaction from hydroxypivaldehyde in the presence of a basic catalyst (e.g., aluminium oxide).[6]
- The Tishchenko reaction of paraformaldehyde in the presence of aluminum methylate or magnesium methylate forms methyl formate.[7]
- Paraformaldehyde reacts with boric acid to form methyl formate.[8] The key step in the reaction mechanism for this reaction is a 1,3-hydride shift in the hemiacetal intermediate formed from two successive nucleophilic addition reactions, the first one from the catalyst. The hydride shift regenerates the alkoxide catalyst.
Related reactions
- Aldol–Tishchenko reaction
- Baylis–Hillman reaction
- Cannizzaro reaction
- Meerwein–Ponndorf–Verley reduction
- Oppenauer oxidation
References
- ↑ See:
- V. E. Tishchenko (1906), "О действии алкоголятов алюминия на альдегиды. Сложного-эфира конденсации, как новый вид уплотнения альдегида." [On the effect of aluminium alkoxides on aldehydes. Ester condensation, as a new kind of aldehyde condensation.], Журнал Русского Физико-Химического Общества (Journal of the Russian Physico-Chemical Society), 38: 355–418 ; 482–540.
- В. Е. Тищенко and Г. Н. Григорьева (V. E. Tishchenko and G. N. Grigor'eva) (1906) "О действии амальгамы магния на изомасляного альдегида" (On the effect of magnesium amalgam on isobutyric aldehyde), Журнал Русского Физико-Химического Общества (Journal of the Russian Physico-Chemical Society), 38 : 540–547.
- М. П. Воронҝова and В. Е. Тищенко (M. P. Voronkova and V. E. Tishchenko) (1906) "О действии амальгамы магния на уксусный альдегид" (On the effect of magnesium amalgam on acetic aldehyde), Журнал Русского Физико-Химического Общества (Journal of the Russian Physico-Chemical Society), 38 : 547–550.
- ↑ В. Тищенко (V. Tishchenko) (1899) "Действие амальгамированного алюминия на алкоголь. Алкоголятов алюминия, их свойства и реакции." (Effect of amalgamated aluminium on alcohol. Aluminium alkoxides, their properties and reactions.), Журнал Русского Физико-Химического Общества (Journal of the Russian Physico-Chemical Society), 31 : 694–770. (in Russian)
- ↑ Kamm, O.; Kamm, W. F. (1941). "Benzyl benzoate". Org. Synth.; Coll. Vol., 1, p. 104
- ↑ Marc Eckert; Gerald Fleischmann; Reinhard Jira; Hermann M. Bolt; Klaus Golka (2007), "Acetaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 4
- ↑ Boy Cornils; Richard W. Fischer; Christian Kohlpaintner (2007), "Butanals", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 4
- ↑ Peter Werle; Marcus Morawietz (2007), "Alcohols, Polyhydric", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 6
- ↑ Günther Reuss; Walter Disteldorf; Armin Otto Gamer; Albrecht Hilt (2007), "Formaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 5
- ↑ Paul R. Stapp (1973). "Boric acid catalyzed Tishchenko reactions". J. Org. Chem. 38 (7): 1433–1434. doi:10.1021/jo00947a049.
This article is issued from Wikipedia - version of the 9/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.