Thulium(III) chloride
 | |
| Names | |
|---|---|
| IUPAC name
Thulium(III) chloride | |
| Other names
Thulium chloride, thulium trichloride | |
| Identifiers | |
| 13537-18-3 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.033.535 |
| EC Number | 236-904-9 |
| PubChem | 61643 |
| RTECS number | XP0525000 |
| |
| |
| Properties | |
| TmCl3 | |
| Molar mass | 275.292 g/mol |
| Appearance | yellow crystals |
| Density | 3.98 g/cm3 |
| Melting point | 824 °C (1,515 °F; 1,097 K) |
| Boiling point | 1,490 °C (2,710 °F; 1,760 K) |
| heptahydrate: very soluble | |
| Solubility | heptahydrate: very soluble in ethanol[1] |
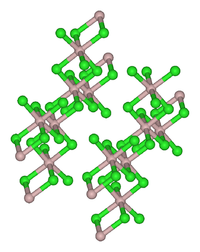
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| 6[2] | |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
966.6 kJ/mol[3] |
| Hazards | |
| Main hazards | Xi (Irritant) |
| S-phrases | S26, S36[4] |
| Related compounds | |
| Other anions |
Thulium(III) oxide |
| Other cations |
Erbium(III) chloride Ytterbium(III) chloride Thulium(II) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Thulium(III) chloride or thulium trichloride is the chemical compound composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions.[5]
Reactions
The hydrated form of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid.[1] Thulium(III) chloride reacts with strong bases to make thulium(III) oxide.
References
- 1 2 Spencer, James F. (1919). "The Metals of the Rare Earths". New York: Longmans, Green, and Co: 152. Retrieved 2008-06-27.
- ↑ "Chemistry: Periodic Table: Thulium: compound data (thulium (III) chloride)". WebElements. Retrieved 2008-06-27.
- ↑ Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of Inorganic Compounds. CRC Press. p. 512. ISBN 0-8493-8671-3. Retrieved 2008-06-27.
- ↑ "439649 Thulium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. Retrieved 2008-06-27.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
This article is issued from Wikipedia - version of the 9/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.