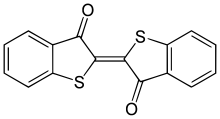
Thioindigo
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-(3-Oxo-1-benzothiophen-2(3H)-ylidene)-1-benzothiophen-3(2H)-one | |
| Other names
DyStar, C.I. Vat Red 41, C.I. 73 300 | |
| Identifiers | |
| 522-75-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4527286 |
| ECHA InfoCard | 100.007.580 |
| PubChem | 3033973 |
| |
| |
| Properties | |
| C16H8O2S2 | |
| Molar mass | 296.36 g·mol−1 |
| Appearance | Red solid |
| Melting point | 280 °C (536 °F; 553 K) |
| Insoluble | |
| Solubility in ethanol, xylene | Soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Thioindigo is an organosulfur compound that is used to dye polyester fabric. A synthetic dye, thioindigo is related to the plant-derived dye indigo, replacing two NH groups with two sulfur atoms.
Thioindigo is generated by the alkylation of the sulfur in thiosalicylic acid with chloroacetic acid. The resulting thioether cyclizes to 2-hydroxythianaphthene, which is easily converted to thioindigo.[1] The related compound 4,7,4',7'-tetrachlorothioindigo, also a commercially important dye, can be prepared by chlorination of thioindigo.
References
- ↑ Elmar Steingruber "Indigo and Indigo Colorants" in Ullmann's Encyclopedia of Industrial Chemistry, 2004, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a14_149.pub2
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.