Theta defensin
| θ-defensin | |
|---|---|
 | |
| Identifiers | |
| Symbol | N/A |
| OPM superfamily | 226 |
| OPM protein | 2atg |
Theta-defensins (θ-defensins. retrocyclins) are a family of mammalian antimicrobial peptides.[1] They are found in non-human 'Old World' primates, but not in human, gorilla, bonobo, and chimpanzee.[2][3]
Structure

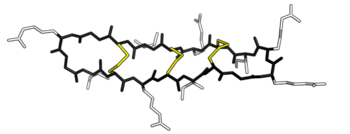

θ-defensins are cyclic peptides of 18 amino acids (~2 kDa), possessing antimicrobial activity against a range of Gram-positive and Gram-negative bacteria, fungi, and some retroviruses.[4][5][6][7] They consist of a pair of antiparallel β-sheets linked by three disulphide bonds arranged as a ladder along the sheets to form an extremely stable structure. Additionally, the peptides may self-associate into trimers.[8]
Biosynthesis
In rhesus macaque (Macaca mulatta) and olive baboon (Papio anubis), θ-defensins are produced from 76 amino acid precursor proteins. A single nine amino acid peptide is derived from each precursor. Two of these nine amino acid peptides are spliced together to form the circular 18 amino acid defensin.[4][5][6][7] Since there are two precursor genes (rhesus theta defensin RTD-1 and RTD-2) they can form 3 different mature θ-defensins: the homodimer of processed RTD-1, The homodimer of processed RTD-2 or the heterodimer composed of both precursors. The heterodimeric form is the most abundant.
In the olive baboon, four θ-defensin precursor genes have been isolated: BTD-a, BTD-b, BTD-c and BTD-d, which encode subunits A, B, C and D. These four subunits could theoretically combine to produce 10 different processed defensins. However, only five have been observed: consisting of subunits A+A, A+B, A+C, A+D and B+B (Referred to as BTD-3, BTD-1, BTD-4, BTD7 and BTD-2 respectively).[7] Finally, orangutan genomes encode 4 θ-defensin precursor genes and gibbon genomes encode 2.
Activity
Antimicrobial activity has been reported against:
Antimicrobial activity is generally through binding to and disrupting the cell membrane. Antiviral activity appears to derive from their binding to the sugar component of glycoproteins and blocking the entry of viruses into the cell.[9]
Evolution
Old World monkey defensins
θ-defensins are extremely divergent members of the defensin protein superfamily which includes alpha-, beta- and big-defensins. The θ-defensins appear to have evolved from α-defensin genes around 40 million years ago in Old World monkeys. The genes were later inactivated in the ancestor of chimpanzees, bonobos, gorillas and humans by mutation that introduced a premature stop codon.[3]
Human retrocyclin pseudogenes
Although New World monkeys and great apes do not produce θ-defensin proteins, human genomes do encode θ-defensin genes which are transcribed to mRNA but not translated due to a premature stop codon.[10] When the mature defensin that would be expressed is chemically synthesised in a laboratory, it shows antimicrobial activity (including anti-retrovirus, leading to the name retrocyclin).[11] It is unknown whether this pseudogenisation gave a selective advantage or was due to genetic drift. The anti-HIV activity of retrocyclins has been further enhanced by protein engineering efforts with the aim of generating a viable treatment.
See also
References
- ↑ Conibear AC, Craik DJ (26 September 2014). "The chemistry and biology of theta defensins.". Angewandte Chemie International Edition in English. 53 (40): 10612–23. doi:10.1002/anie.201402167. PMID 25079086.
- ↑ Cole AM, Wang W, Waring AJ, Lehrer RI (October 2004). "Retrocyclins: using past as prologue". Curr. Protein Pept. Sci. 5 (5): 373–81. doi:10.2174/1389203043379657. PMID 15544532.
- 1 2 Li D, Zhang L, Yin H, Xu H, Satkoski Trask J, Smith DG, Li Y, Yang M, Zhu Q (Jun 2014). "Evolution of primate α and θ defensins revealed by analysis of genomes". Molecular biology reports. 41 (6): 3859–66. doi:10.1007/s11033-014-3253-z. PMID 24557891.
- 1 2 Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME (October 1999). "A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins". Science. 286 (5439): 498–502. doi:10.1126/science.286.5439.498. PMID 10521339.
- 1 2 Leonova L, Kokryakov VN, Aleshina G, Hong T, Nguyen T, Zhao C, Waring AJ, Lehrer RI (September 2001). "Circular minidefensins and posttranslational generation of molecular diversity". J. Leukoc. Biol. 70 (3): 461–4. PMID 11527997.
- 1 2 Tran D, Tran PA, Tang YQ, Yuan J, Cole T, Selsted ME (February 2002). "Homodimeric θ-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides". J. Biol. Chem. 277 (5): 3079–3084. doi:10.1074/jbc.M109117200. PMID 11675394.
- 1 2 3 Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME (December 2008). "Isolation, synthesis, and antimicrobial activities of naturally occurring θ-defensin isoforms from baboon leukocytes". Infect. Immun. 76 (12): 5883–5891. doi:10.1128/IAI.01100-08. PMC 2583559
 . PMID 18852242.
. PMID 18852242. - ↑ Daly NL, Chen YK, Rosengren KJ, Marx UC, Phillips ML, Waring AJ, Wang W, Lehrer RI, Craik DJ (Sep 4, 2007). "Retrocyclin-2: structural analysis of a potent anti-HIV theta-defensin". Biochemistry. 46 (35): 9920–8. doi:10.1021/bi700720e. PMID 17685559.
- ↑ Cascales L, Craik DJ (Nov 21, 2010). "Naturally occurring circular proteins: distribution, biosynthesis and evolution.". Organic & biomolecular chemistry. 8 (22): 5035–47. doi:10.1039/c0ob00139b. PMID 20835453.
- ↑ Venkataraman N, Cole AL, Ruchala P, Waring AJ, Lehrer RI, Stuchlik O, Pohl J, Cole AM (Apr 28, 2009). "Reawakening retrocyclins: ancestral human defensins active against HIV-1.". PLOS Biology. 7 (4): e95. doi:10.1371/journal.pbio.1000095. PMC 2672613
 . PMID 19402752.
. PMID 19402752. - ↑ Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI (Feb 19, 2002). "Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1.". Proceedings of the National Academy of Sciences of the United States of America. 99 (4): 1813–8. doi:10.1073/pnas.052706399. PMID 11854483.