Stigmatellin
 | |
| Identifiers | |
|---|---|
| 91682-96-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:32155 |
| ChEMBL | ChEMBL486556 |
| ChemSpider | 394850 |
| ECHA InfoCard | 100.149.842 |
| MeSH | Stigmatellin |
| PubChem | 447884 |
| |
| |
| Properties | |
| C30H42O7 | |
| Molar mass | 514.65 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Stigmatellin is a potent inhibitor of the quinol oxidation (Qo) site of the cytochrome bc1 complex in mitochondria and the cytochrome b6f complex of thylakoid membranes.
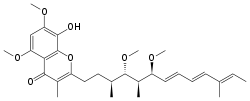
Stigmatellin is isolated from the myxobacterium Stigmatella aurantica, and contains a 5,7-dimethoxy-8-hydroxychromone aromatic headgroup with a hydrophobic alkenyl chain in position 2 . Crystal structures for stigmatellin-inhibited bovine and yeast (Saccharomyces cerevisiae) bc1 complex are available. Stigmatellin binds at the cytochrome b Qo site in the '(heme) bl distal' position, and associates with the Rieske iron-sulfur protein via a hydrogen bond to histidine residue 181 (His-181), a ligand to the [2Fe2S] iron-sulfur cluster of this subunit. This association raises the midpoint potential of the iron-sulfur cluster from 290 to 540 mV and restricts movement of the cytoplasmic domain of the Rieske protein.
References
- von Jagow, G., and Link, T.A. Methods in Enzymology 126: 253-271 (1986)