Sodium metavanadate
 | |
| Names | |
|---|---|
| IUPAC name
Sodium trioxovanadate(V) | |
| Identifiers | |
| 13718-26-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75221 |
| ECHA InfoCard | 100.033.869 |
| EC Number | 237-272-7 |
| PubChem | 4148882 |
| RTECS number | YW1050000 |
| |
| |
| Properties | |
| NaVO3 | |
| Molar mass | 121.9295 g/mol |
| Appearance | yellow crystalline solid |
| Density | 5.15 g/cm3 |
| Melting point | 630 °C (1,166 °F; 903 K) |
| 19.3 g/100 mL (20 °C) 40.8 g/100 mL (80 °C) | |
| Thermochemistry | |
| 97.6 J/mol K | |
| Std molar entropy (S |
113.8 J/mol K |
| Std enthalpy of formation (ΔfH |
−1148 kJ/mol |
| Hazards | |
| Main hazards | Toxic, irritant |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
98 mg/kg (rat, oral) |
| Related compounds | |
| Other anions |
Sodium orthovanadate |
| Other cations |
Ammonium metavanadate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

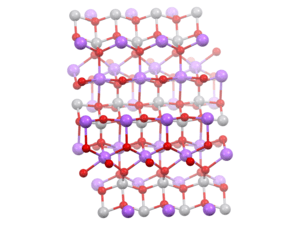
Chain of vanadate VO4 tetrahedral units, each sharing two corners
Sodium metavanadate is the inorganic compound with the formula NaVO3.[1] It is a yellow, water-soluble solid. Its natural forms include mineral metamunirite (anhydrous) and a dihydrate, munirite. Both are very rare, metamunirite is now known only from vanadium- and uranium-bearing sandstone formations of central-western USA and munirite from Pakistan and South Africa.[2]
References
- ↑ Kato, K.; Takayama, E. (1984). "Das Entwässerungsverhalten des Natriummetavanadatdihydrats und die Kristallstruktur des beta-Natriummetavanadats" [The dehydration activity of sodium metavanadate dihydrate and the crystal structure of β-sodium metavanadate]. Acta Crystallogr. B40: 102–105. doi:10.1107/S0108768184001828.
- ↑ "Munirite". Mindat.
This article is issued from Wikipedia - version of the 6/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
