RAGE (receptor)
| View/Edit Human | |||
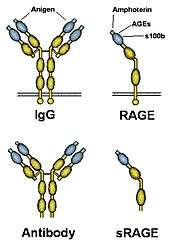
RAGE, the receptor for advanced glycation endproducts is a 35 kDa transmembrane receptor of the immunoglobulin super family which was first characterized in 1992 by Neeper et al.[2] It is also called "AGER". Its name comes from its ability to bind advanced glycation endproducts (AGE), which include chiefly glycoproteins, the glycans of which have been modified non-enzymatically through the Maillard reaction. In view of its inflammatory function in innate immunity and its ability to detect a class of ligands through a common structural motif, RAGE is often referred to as a pattern recognition receptor. RAGE also has at least one other agonistic ligand: high mobility group protein B1 (HMGB1). HMGB1 is an intracellular DNA-binding protein important in chromatin remodeling which can be released by necrotic cells passively and by active secretion from macrophages, natural killer (NK) cells and dendritic cells.
The interaction between RAGE and its ligands is thought to result in pro-inflammatory gene activation.[3] Due to an enhanced level of RAGE ligands in diabetes or other chronic disorders, this receptor is hypothesised to have a causative effect in a range of inflammatory diseases such as diabetic complications, Alzheimer's disease and even some tumors.
Isoforms of the RAGE protein, which lack the transmembrane and the signaling domain (commonly referred to as soluble RAGE or sRAGE) are hypothesized to counteract the detrimental action of the full-length receptor and are hoped to provide a means to develop a cure against RAGE-associated diseases.
Gene and polymorphisms
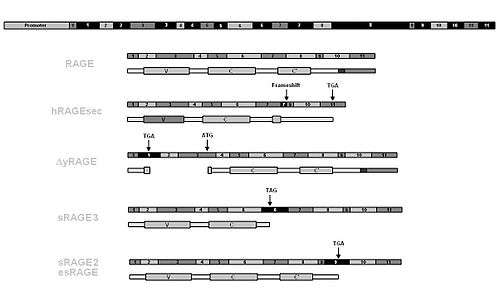
The human RAGE gene lies within the major histocompatibility complex (MHC) class III region on chromosome 6 and comprises 11 exons interlaced by 10 introns. Total length of the gene is about 1400 base pairs (bp) including the promoter region, which partly overlaps with the PBX2 gene.[4] About 30 polymorphisms are known most of which are single nucleotide polymorphisms (SNP).[5]
RNA and alternative splicing
The primary transcript of the human RAGE gene (pre-mRNA) is thought to be alternatively spliced. So far about 6 isoforms including the full length transmembrane receptor have been found in different tissues such as lung, kidney, brain etc. Five of these 6 isoforms lack the transmembrane domain and are thus believed to be secreted from cells. Generally these isoforms are referred to as sRAGE (soluble RAGE) or esRAGE (endogenous secretory RAGE). One of the isoforms lacks the V-domain and is thus believed not to be able to bind RAGE ligands.
Structure
The full receptor consists of 5 domains: The cytosolic domain, which is responsible for signal transduction, the transmembrane domain which anchors the receptor in the cell membrane, the variable domain which binds the RAGE ligands, and two constant domains.
Ligands
RAGE is able to bind several ligands and therefore is referred to as a pattern-recognition receptor. Ligands which have so far been found to bind RAGE are:
- AGE
- HMGB1 (Amphoterin)
- S100A12 (EN-RAGE)
- S100B
- S100A7 (psoriasin) but not highly homologous S100A15 (koebnerisin)
- S100P[6][7]
- S100A8/A9 complex referred to as calprotectin[8]
- Amyloid-β-protein
- Mac-1
- Phosphatidylserine.
RAGE and disease
RAGE has been linked to several chronic diseases, which are thought to result from vascular damage. The pathogenesis is hypothesized to include ligand binding, upon which RAGE signals activation of nuclear factor kappa B (NF-κB). NF-κB controls several genes involved in inflammation. RAGE itself is upregulated by NF-κB. Given a condition in which there is a large amount of RAGE ligands (e.g. AGE in diabetes or amyloid-β-protein in Alzheimer's disease) this establishes a positive feed-back cycle, which leads to chronic inflammation. This chronic condition is then believed to alter the micro- and macrovasculature, resulting in organ damage or even organ failure. Diseases that have been linked to RAGE are:
- Atherosclerosis
- Peripheral vascular disease
- Myocardial infarction
- Congestive heart failure
- Diabetic retinopathy
- Diabetic neuropathy
- Diabetic nephropathy
- Alzheimer's disease
- Psoriasis
- Takayasu's arteritis[9]
RAGE is expressed at the highest levels in the lung compared to other tissues, in particular in alveolar epithelial type I cells, and is lost in Idiopathic pulmonary fibrosis (IPF) indicating that expression and regulation of RAGE in the pulmonary system differs from that in the vascular system. Blockade/knockdown of RAGE resulted in impaired cell adhesion, and increased cell proliferation and migration[10]
Inhibitors
A number of small molecule RAGE inhibitors or antagonists have been reported.[11][12][13][14] vTv Therapeutics (formerly TransTech Pharma) is sponsoring a Phase 3 clinical trial of their RAGE inhibitor Azeliragon (TTP488) for mild Alzheimer's disease.[15][16]
AGE receptors
Besides RAGE there are other receptors which are believed to bind advanced glycation endproducts. However, these receptors could play a role in removal of AGE rather than in signal transduction as it is the case for RAGE. Other AGE receptors are:
- SR-A (Macrophage scavenger receptor Type I and II)
- OST-48 (Oligosaccharyl transferase-4) (AGE-R1)
- 80 K-H phosphoprotein (Proteinkinase C substrate) (AGE-R2)
- Galectin-3 (AGE-R3)
- LOX-1 (Lectin-like oxidized low density lipoprotein receptor-1)
- CD36
References
- ↑ "Human PubMed Reference:".
- ↑ Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A (Jul 1992). "Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins". The Journal of Biological Chemistry. 267 (21): 14998–5004. PMID 1378843.
- ↑ Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP (Dec 2001). "Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB". Diabetes. 50 (12): 2792–808. doi:10.2337/diabetes.50.12.2792. PMID 11723063.
- ↑ Hudson BI, Stickland MH, Futers TS, Grant PJ (Jun 2001). "Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy". Diabetes. 50 (6): 1505–11. doi:10.2337/diabetes.50.6.1505. PMID 11375354.
- ↑ Hudson BI, Hofman MA, Bucciarelli L, Wendt T, Moser B, Lu Y, Qu W, Stern DM, D'Agati V, Yan SD, Yan SF, Grant PJ (2002). "Glycation and diabetes: The RAGE connection" (PDF). Current Science. 83 (12): 1515–1521.
- ↑ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-08-01). "Structural Insights into Calcium-Bound S100P and the V Domain of the RAGE Complex". PLoS ONE. 9 (8). doi:10.1371/journal.pone.0103947. ISSN 1932-6203. PMC 4118983
 . PMID 25084534.
. PMID 25084534. - ↑ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-10-17). "Interaction between S100P and the anti-allergy drug cromolyn". Biochemical and Biophysical Research Communications. 454 (3): 404–409. doi:10.1016/j.bbrc.2014.10.048. ISSN 1090-2104. PMID 25450399.
- ↑ Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D (Jan 2006). "S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells". Experimental Cell Research. 312 (2): 184–97. doi:10.1016/j.yexcr.2005.10.013. PMID 16297907.
- ↑ Mahajan N, Mahmood S, Jain S, Dhawan V (Sep 2013). "Loss of RAGE in Pulmonary Fibrosis". International Journal of Cardiology. 168 (1): 532–4. doi:10.1016/j.ijcard.2013.01.002. PMID 23398829.
- ↑ Queisser MA, Kouri FM, Königshoff M, Wygrecka M, Schubert U, Eickelberg O, Preissner KT (Sep 2008). "Loss of RAGE in Pulmonary Fibrosis". American Journal of Respiratory Cell and Molecular Biology. 39 (3): 5337–45. doi:10.1165/rcmb.2007-0244OC. PMID 18421017.
- ↑ Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV (Apr 2012). "A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease". The Journal of Clinical Investigation. 122 (4): 1377–92. doi:10.1172/JCI58642. PMID 22406537.
- ↑ Han YT, Choi GI, Son D, Kim NJ, Yun H, Lee S, Chang DJ, Hong HS, Kim H, Ha HJ, Kim YH, Park HJ, Lee J, Suh YG (Nov 2012). "Ligand-based design, synthesis, and biological evaluation of 2-aminopyrimidines, a novel series of receptor for advanced glycation end products (RAGE) inhibitors". Journal of Medicinal Chemistry. 55 (21): 9120–35. doi:10.1021/jm300172z. PMID 22742537.
- ↑ Han YT, Kim K, Choi GI, An H, Son D, Kim H, Ha HJ, Son JH, Chung SJ, Park HJ, Lee J, Suh YG (May 2014). "Pyrazole-5-carboxamides, novel inhibitors of receptor for advanced glycation end products (RAGE)". European Journal of Medicinal Chemistry. 79: 128–42. doi:10.1016/j.ejmech.2014.03.072. PMID 24727489.
- ↑ Han YT, Kim K, Son D, An H, Kim H, Lee J, Park HJ, Lee J, Suh YG (Feb 2015). "Fine tuning of 4,6-bisphenyl-2-(3-alkoxyanilino)pyrimidine focusing on the activity-sensitive aminoalkoxy moiety for a therapeutically useful inhibitor of receptor for advanced glycation end products (RAGE)". Bioorganic & Medicinal Chemistry. 23 (3): 579–87. doi:10.1016/j.bmc.2014.12.003. PMID 25533401.
- ↑ "Azeliragon". vTv Therapeutics. vTv Therapeutics. Retrieved 23 July 2015.
- ↑ "Evaluation of the Efficacy and Safety of Azeliragon (TTP488) in Patients With Mild Alzheimer's Disease (STEADFAST)". ClinicalTrials. NIH. Retrieved 23 July 2015.
Further reading
- Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM (Aug 2004). "RAGE axis: Animal models and novel insights into the vascular complications of diabetes". Arteriosclerosis, Thrombosis, and Vascular Biology. 24 (8): 1342–9. doi:10.1161/01.ATV.0000133191.71196.90. PMID 15155381.
- Simm A, Bartling B, Silber RE (Jun 2004). "RAGE: a new pleiotropic antagonistic gene?". Annals of the New York Academy of Sciences. 1019: 228–31. doi:10.1196/annals.1297.038. PMID 15247020.
- Nawroth P, Bierhaus A, Marrero M, Yamamoto H, Stern DM (Feb 2005). "Atherosclerosis and restenosis: is there a role for RAGE?". Current Diabetes Reports. 5 (1): 11–6. doi:10.1007/s11892-005-0061-9. PMID 15663911.
External links
- RAGE receptor at the US National Library of Medicine Medical Subject Headings (MeSH)
- AGER on the Atlas of Genetics and Oncology