Quinone methide
Quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylene group (a double bonded carbon). The carbonyl and methylene are usually oriented either ortho or para to each other (there are some examples of transient synthetic meta quinone methides). The two simplest examples of this class of compounds are pictured below.

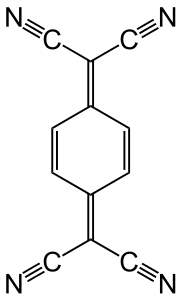
Quinone dimethides are related derivatives of quinone wherein both oxo groups are replaced by methylene or substituted methylene groups. A well studied examine is tetracyanoquinodimethane.
Properties
Quinone methides are structurally related to quinones with one of the carbonyl oxygens replaced by a methylene group. This structural change makes the molecule much more polarized and thus more reactive.
Simple quinone methides are short lived intermediates that are not stable enough to be isolated under normal circumstances but quickly react with nucleophiles and other reactants.
Some quinone methides have structural (e.g. steric hindrance) or electronic characteristics that stabilize them enough to be isolated.
Occurrence and applications
Quinone methanide arises by the degradation of tyrosine, leading ultimately to p-cresol.[1]
Quinones methides and their derivatives are common constituents of biological systems. They are reactive intermediates directly involved in the process of lignification (creation of complex lignin polymers) in plants.[2]
Many quinone methides show pronounced biological activity. They have been implicated as the ultimate cytotoxins responsible for the effects of such agents as antitumor drugs, antibiotics, and DNA alkylators.[3] Oxidation to a reactive quinone methide is the mechanistic basis of many phenolic anti-cancer drugs.
Celastrol is a triterpenoid quinone methide isolated from Tripterygium wilfordii (Thunder of God vine) and Celastrus regelii that exhibits antioxidant (15 times the potency of α-tocopherol),[4] anti-inflammatory,[5] anticancer,[6][7][8][9] and insecticidal [10] activities.

Pristimerin, the methyl ester of celasterol, is a triterpenoid quinone methide isolated from Maytenus heterophylla that displays antitumor and antiviral [11] activities.

Taxodone and its oxidized rearrangement product, taxodione, are diterpenoid quinone methides found in Taxodium distichum (bald cypress), Rosmarinus officinalis (rosemary), several Salvia species and other plants, that display anticancer,[12][13][14] antibacterial,[15][16][17] antioxidant,[18] antifungal,[19] insecticide,[20] and antifeedant [21] activities.

Kendomycin is an antitumor antibacterial quinone methide macrolide first isolated from the bacterium Streptomyces violaceoruber.[22] It has potent activity as an endothelin receptor antagonist and anti-osteoporosis agent.[23]

Elansolid A3 is a quinone methide from the bacterium Chitinophaga sancti that displays antibiotic activity.[24] Antibacterial quinone methides, 20-epi-isoiguesterinol, 6-oxoisoiguesterin, isoiguesterin and isoiguesterinol were found in Salacia madagascariensis.[25] Quinone methides tingenone and netzahualcoyonol were isolated from Salacia petenensis.[26] Nortriterpenoid quinone methide amazoquinone and (7S, 8S)-7-hydroxy-7,8-dihydro-tingenone were isolated from Maytenus amazonica.[27] An antimicrobrial quinone methide, 15 alpha-hydroxypristimerin, was isolated from a South American medicinal plant, Maytenus scutioides.[28] Maytenoquinone, an isomer of taxodione, is a biologically active quinone methide found in Maytenus dispermus.[29]

Preparation
Quinone methides are often prepared by oxidation of the corresponding ortho or para phenol toluene.
Quinone methides can be produced in aqueous solution by photochemical dehydration of o-hydroxybenzyl alcohols.
Reactions
Quinone methides are electrophilic Michael acceptors that generally react quickly with nucleophiles, other reactants, and are readily reduced. Quinone methides are conjugated but not aromatic. Conjugate addition usually breaks the conjugation. Reduction can either rearomatise the compound or break the conjugation.
See also
References
- ↑ Stich, T. A.; Myers, W. K.; Britt, R. D., "Paramagnetic intermediates generated by radical S-adenosylmethionine (SAM) enzymes", Acc. Chem. Res. 2014, 47, 2235-2243.
- ↑ Quinone Methides in Lignification
- ↑ Wang P, Song Y, Zhang L, He H, Zhou X (2005). "Quinone methide derivatives: important intermediates to DNA alkylating and DNA cross-linking actions". Curr Med Chem. 12 (24): 2893–2913. doi:10.2174/092986705774454724. PMID 16305478.
- ↑ Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C (2001). "Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease.". Prog Neuropsychopharmacol Biol Psychiatry. 25 (7): 1341–1357. doi:10.1016/S0278-5846(01)00192-0. PMID 11513350.
- ↑ Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK (2009). "Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii". Eur J Clin Invest. 39 (9): 819–827. doi:10.1111/j.1365-2362.2009.02186.x. PMID 19549173.
- ↑ Lee JH, Choi KJ, Seo WD, Jang SY, Kim M, Lee BW, Kim JY, Kang S, Park KH, Lee YS, Bae S (2011). "Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90.". Int J Mol Med. 27 (3): 441–446. doi:10.3892/ijmm.2011.601. PMID 21249311.
- ↑ Tiedemann; et al. (2009). "Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo.". Blood. 113 (17): 4027–37. doi:10.1182/blood-2008-09-179796. PMID 19096011.
- ↑ Zhu H, Liu XW, Cai TY, Cao J, Tu CX, Lu W, He QJ, Yang B (2010). "Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway". J Pharmacol Exp Ther. 334 (2): 489–499. doi:10.1124/jpet.110.165654. PMID 20472666.
- ↑ Byun; et al. (2009). "Reactive oxygen species-dependent activation of Bax and Poly(ADP)-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid Pristimerin in human cervical cancer cells.". Mol.Pharmacol. 76 (4): 734–44. doi:10.1124/mol.109.056259. PMID 19574249.
- ↑ Avilla J, Teixidò A, Velázquez C, Alvarenga N, Ferro E, Canela R (2000). "Insecticidal activity of Maytenus species (Celastraceae) nortriterpene quinone methides against codling moth, Cydia pomonella (L.) (Lepidoptera: tortricidae).". Journal of Agricultural and Food Chemistry. 48 (1): 88–92. doi:10.1021/jf990008w. PMID 10637057.
- ↑ Murayama T, Eizuru Y, Yamada R, Sadanari H, Matsubara K, Rukung G, Tolo FM, Mungai GM, Kofi-Tsekpo M (2007). "Anticytomegalovirus activity of pristimerin, a triterpenoid quinone methide isolated from Maytenus heterophylla (Eckl. & Zeyh.).". Antivir Chem Chemother. 18 (3): 133–139. PMID 17626597.
- ↑ Kupchan, S. M.; Karim, A; Marcks, C. (1968). "Tumor inhibitors. XXXIV. Taxodione and taxodone, two novel diterpenoid quinone methide tumor inhibitors from Taxodium distichum". J Am Chem Soc. 90 (21): 5923. doi:10.1021/ja01023a061.
- ↑ Zaghloul AM, Gohar AA, Naiem ZA, Abdel Bar FM (2008). "Taxodione, a DNA-binding compound from Taxodium distichum L. (Rich.).". Z Naturforsch C. 63 (5-6): 355–360.
- ↑ Ayhan Ulubelen, Gülaçti Topçu, Hee-Byung Chai and John M. Pezzuto (1999). "Cytotoxic Activity of Diterpenoids Isolated from Salvia hypargeia". Pharmaceutical Biology. 37 (2): 148–151. doi:10.1076/phbi.37.2.148.6082.
- ↑ Vivek K. Bajpai & Sun Chul Kan (2010). "Antibacterial abietane-type diterpenoid, taxodone from Metasequoia glyptostroboides Miki ex Hu". Journal of Bioscience. 35 (4): 533–538. doi:10.1007/s12038-010-0061-z.
- ↑ Vivek K. Bajpai; Minkyun Na; Sun Chul Kang (2010). "The role of bioactive substances in controlling foodborne pathogens derived from Metasequoia glyptostroboides Miki ex Hu". Food and Chemical Toxicology. 48: 1945–1949. doi:10.1016/j.fct.2010.04.041.
- ↑ Tada M, Kurabe J, Yoshida T, Ohkanda T, Matsumoto Y (2010). "Syntheses and antibacterial activities of diterpene catechol derivatives with abietane, totarane and podocarpane skeletons against methicillin-resistant Staphylococcus aureus and Propionibacterium acnes". Chem Pharm Bull. 58 (6): 818–824. doi:10.1248/cpb.58.818.
- ↑ Ufuk Kolak; Ahmed Kabouche; Mehmet Öztürk; Zahia Kabouche; Gülaçtl Topçu; Ayhan Ulubelen (2009). "Antioxidant diterpenoids from the roots of Salvia barrelieri". Phytochemical Analysis. 20 (4): 320–327. doi:10.1002/pca.1130.
- ↑ Norihisa Kusumoto, Tatsuya Ashitani, Tetsuya Murayama, Koichi Ogiyama and Koetsu Takahashi (2010). "Antifungal Abietane-Type Diterpenes from the Cones of Taxodium distichum Rich". Journal of Chemical Ecology. 36 (12): 1381–1386. doi:10.1007/s10886-010-9875-2.
- ↑ Norihisa Kusumoto, Tatsuya Ashitani, Yuichi Hayasaka, Tetsuya Murayama, Koichi Ogiyama and Koetsu Takahashi (2009). "Antitermitic Activities of Abietane-type Diterpenes from Taxodium distichum Cones". Journal of Chemical Ecology. 35 (6): 635–642. doi:10.1007/s10886-009-9646-0.
- ↑ M. C. Ballesta-Acosta1, M. J. Pascual-Villalobos and B. Rodríguez (2008). "Short communication. The antifeedant activity of natural plant products towards the larvae of Spodoptera littoralis". Spanish Journal of Agricultural Research. 6 (1): 85–91. doi:10.5424/sjar/2008061-304.
- ↑ H B Bode & A Zeeck (2000). "Structure and biosynthesis of kendomycin, a carbocyclic ansa-compound from Streptomyces". J Chem Soc Perkin Trans 1. 323: 323. doi:10.1039/a908387a.
- ↑ Burke Research Group University of Wisconsin
- ↑ Jansen R, Gerth K, Steinmetz H, Reinecke S, Kessler W, Kirschning A, Müller R (2011). "Elansolid A3, a Unique p-Quinone Methide Antibiotic from Chitinophaga sancti.". Chemistry. 17 (28): 7739–44. doi:10.1002/chem.201100457. PMID 21626585.
- ↑ Thiem DA, Sneden AT, Khan SI, Tekwani BL (2005). "Bisnortriterpenes from Salacia madagascariensis.". J Nat Prod. 68 (2): 251–254. doi:10.1021/np0497088.
- ↑ Setzer WN, Holland MT, Bozeman CA, Rozmus GF, Setzer MC, Moriarity DM, Reeb S, Vogler B, Bates RB, Haber WA (2001). "Isolation and frontier molecular orbital investigation of bioactive quinone-methide triterpenoids from the bark of Salacia petenensis.". Planta Med. 67 (1): 65–69. doi:10.1055/s-2001-10879. PMID 11270725.
- ↑ Chávez H, Estévez-Braun A, Ravelo AG, González AG (1999). "New phenolic and quinone-methide triterpenes from Maytenus amazonica.". J Nat Prod. 62 (3): 434–436. doi:10.1021/np980412+. PMID 10096852.
- ↑ González AG, Alvarenga NL, Bazzocchi IL, Ravelo AG, Moujir L (1998). "A new bioactive norquinone-methide triterpene from Maytenus scutioides.". Planta Med. 64 (8): 767–771. doi:10.1055/s-2006-957581. PMID 10075545.
- ↑ J. D. Martín (1973). "New diterpenoids extractives of Maytenus dispermus". Tetrahedron. 29 (17): 2553–2559. doi:10.1016/0040-4020(73)80172-3.
External links
- Formation and Stability of Simple Quinone Methides
- Quinone methide intermediates in organic Photochemistry
- Reactive intermediates. Some chemistry of quinone methides
- o-Quinone methides: intermediates underdeveloped and underutilized in organic synthesis