Pterin
 | |
| Names | |
|---|---|
| IUPAC names
2-aminopteridin-4(3H)-one (one of five tautomers) | |
| Other names
Pteridoxamine Pterine 4-Oxopterin 2-Amino-4-pteridone 2-Amino-4-hydroxypteridine 2-Amino-4-oxopteridine 2-aminopteridin-4-ol 2-Amino-4-pteridinol | |
| Identifiers | |
| 2236-60-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:44992 |
| ChEMBL | ChEMBL278009 |
| ChemSpider | 65806 |
| ECHA InfoCard | 100.017.091 |
| PubChem | 73000 |
| |
| |
| Properties | |
| C6H5N5O | |
| Molar mass | 163.137 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Pterin is a heterocyclic compound composed of a pteridine ring system, with a keto group and an amino group on positions 4 and 2 respectively. Several tautomers of pterin exist and are shown below. Derivatives of pterin include pterins and folates.
Pterins, as a group, are compounds that are derivatives of the basic pterin structure, described in the beginning, with additional functional groups attached to the pyrazine subring. Pterins were first discovered in the pigments of butterfly wings[1] (hence the origin of their name, from the Greek pteron (πτερόν),[2] wing) and perform many roles in coloration in the biological world. Pterins also function as cofactors in enzyme catalysis.
Folates are “conjugated” pterins that contain p-aminobenzoic acid (known as pteroic acid) and L-glutamates connected to the methyl group at position 6 of the pteridine ring system. They are critical compounds in a large number of biological group transfer reactions. These folate-dependent biosynthetic reactions include the transfer of methyl groups from 5-methyltetrahydrofolate to homocysteine to form L-methionine, and the transfer of formyl groups from 10-formyltetrahydrofolate to L-methionine to form N-formylmethionine in initiator tRNAs.
Tautomers of pterin
 2-aminopteridin-4(1H)-one
2-aminopteridin-4(1H)-one 2-aminopteridin-4(3H)-one
2-aminopteridin-4(3H)-one 2-aminopteridin-4(8H)-one
2-aminopteridin-4(8H)-one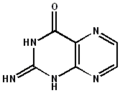 2-imino-2,3-dihydropteridin-4(1H)-one
2-imino-2,3-dihydropteridin-4(1H)-one 2-aminopteridin-4-ol
2-aminopteridin-4-ol
Biosynthesis
The biosynthesis of pterins begins with the molecule guanosine triphosphate (GTP); the enzyme that controls the conversion of GTP to pterin, GTP cyclohydrolase I, is found in both prokaryotes and eukaryotes.
Other pterins
Pterin can exist in many different forms in nature depending on its function. Tetrahydrobiopterin (BH4), the major unconjugated pteridine in vertebrates, is a co-factor in the hydroxylation of aromatic compounds and synthesis of nitric oxide. Molybdopterin is a substituted pteridine that binds molybdenum to yield redox cofactors involved in biological hydroxylations, reduction of nitrate, and respiratory oxidation. Tetrahydromethanopterin is used in methanogenic organisms. Cyanopterin is a glycosylated version of pteridine of unknown function in cyanobacteria.
See also
References
External links
http://grupoargentinodefotobiologia.info/grupos/pteridinas/e_index.html