Pfitzinger reaction
| Pfitzinger reaction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | W. Pfitzinger | ||||||||||||
| Reaction type | ring-condensation | ||||||||||||
| Reaction | |||||||||||||
| |||||||||||||
| Conditions | |||||||||||||
| Typical solvents | protic | ||||||||||||
The Pfitzinger reaction (also known as the Pfitzinger-Borsche reaction) is the chemical reaction of isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids.[1][2]
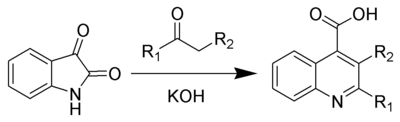
Several reviews have been published.[3][4][5]
Reaction mechanism
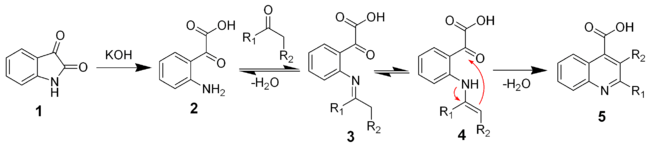
The reaction of isatin with a base such as potassium hydroxide hydrolyses the amide bond to give the keto-acid 2. This intermediate can be isolated, but is typically not. A ketone (or aldehyde) will react with the aniline to give the imine (3) and the enamine (4). The enamine will cyclize and dehydrate to give the desired quiniline (5).
Variations
Halberkann variant
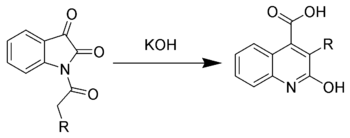
Reaction of N-acyl isatins with base gives 2-hydroxy-quinoline-4-carboxylic acids.[6]
See also
- Camps quinoline synthesis
- Friedländer synthesis
- Niementowski quinazoline synthesis
- Talnetant, Cinchocaine
References
- ↑ Pfitzinger, W. (1886). "Chinolinderivate aus Isatinsäure". J. Prakt. Chem. 33: 100. doi:10.1002/prac.18850330110.
- ↑ Pfitzinger, W. (1888). "Chinolinderivate aus Isatinsäure". J. Prakt. Chem. 38: 582. doi:10.1002/prac.18880380138.
- ↑ Manske, R. H. (1942). Chem. Rev. 30: 126. doi:10.1021/cr60095a006. Missing or empty
|title=(help) - ↑ Bergstrom, F. W. (1944). "Heterocyclic Nitrogen Compounds. Part IIA. Hexacyclic Compounds: Pyridine, Quinoline, and Isoquinoline". Chem. Rev. 35 (2): 152. doi:10.1021/cr60111a001.
- ↑ Shvekhgeimer, M. G.-A. (2004). "The Pfitzinger Reaction". Chemistry of Heterocyclic Compounds. 40 (3): 257. doi:10.1023/B:COHC.0000028623.41308.e5.)
- ↑ Halberkann, J. (1921). "Abkömmlinge der Chininsäure". Chemische Berichte. 54 (11): 3090. doi:10.1002/cber.19210541118.
This article is issued from Wikipedia - version of the 5/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.