Parylene
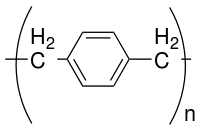
Parylene is the trade name for a variety of chemical vapor deposited poly(p-xylylene) polymers used as moisture and dielectric barriers. Among them, Parylene C is the most popular due to its combination of barrier properties, cost, and other processing advantages.
Parylene is green polymer chemistry. It is self-initiated (no initiator needed) and un-terminated (no termination group needed) with no solvent or catalyst required. The commonly used precursor, [2.2]paracyclophane, yields 100% monomer above 550 °C in vacuum [1] and does not yield any by-products (Gorham Process). There is very little concern that parylene N will be 'over-cracked', meaning [2.2]paracyclophane is converted to p-xylylene cleanly with no side-reactions occurring. However, the same cannot be said for parylene C. The aryl-chlorine bond in dichloro[2.2]paracyclophane readily breaks at 680 °C (standard pyrolysis temperature); and therefore it is desirable to optimize each parylene tool in terms of its pyrolysis temperature using a mass spectrometer.
There are alternative precursors to arrive at the parylene polymers, which possess leaving groups, the most popular using bromine to yield the parylene AF-4 polymer.[2] However, bromine is corrosive towards most metals and metal alloys and Viton O-rings so it is difficult to work with and precautions are needed. More recently, a liquid precursor route was developed yielding parylene N using methoxy leaving group.[3] Although [2.2]paracyclophane is already inexpensive, this precursor is much less expensive and it can delivered reliably using a mass-flow controller (MFC), a huge advantage for process control, which has been lacking for years with the standard Gorham process.
Parylene C and to a lesser extent AF-4, SF, HT (all the same polymer) are used for coating printed circuit boards (PCBs) and medical devices. There are numerous other applications as parylene is an excellent moisture barrier. It is the most bio-accepted coating for stents, defibrillators, pacemakers and other devices permanently implanted into the body.[4]
Parylenes are relatively soft (parylene N 0.5 GPa)[5] except for cross-linked Parylene X (1.0 GPa)[6] and they have poor oxidative resistance (~60-100 °C depending on failure criteria) and UV stability,[7] except for Parylene AF-4. However, Parylene AF-4 is more expensive due to a three-step synthesis of its precursor with low yield and poor deposition efficiency. Their UV stability is so poor that parylene cannot be exposed to regular sunlight without yellowing.
Nearly all the parylenes are insoluble at room temperature except for the alkylated parylenes, one of which is parylene E [8] and the alkylated-ethynyl parylenes.[9] This lack of solubility has made it difficult to re-work printed circuit boards coated with parylene.
Copolymers[10] and nanocomposites (SiO2/parylene C)[11] of parylene have been deposited at near-room temperature previously; and with strongly electron withdrawing comonomers, parylene can be used as an initiator to initiate polymerizations, such as with N-phenyl maleimide. Using the parylene C/SiO2 nanocomposites, parylene C could be used as a sacrificial layer to make nanoporous silica thin films with a porosity of >90%.[12]
Parylene N

Parylene N is a polymer manufactured (chemical vapor deposited) from the p-xylylene intermediate. The p-xylylene intermediate is commonly derived from [2.2]paracyclophane. The latter compound can be synthesized from p-xylene involving several steps involving bromination, amination and Hofmann elimination.[13]
Parylene N is an un-substituted molecule. Heating [2.2]paracyclophane under low pressure (0.01 – 1.0 Torr) conditions and cracking it at 450-700 °C gives rise to the p-xylylene intermediate,[14][15] which polymerizes when physisorbed on a surface. The p-xylylene intermediate has two quantum mechanical states, the benzoid state (triplet state) and the quinoid state (singlet state). The triplet state is effectively the initiator and the singlet state is effectively the monomer. The triplet state can be de-activated when in contact with transition metals or metal oxides including Cu/CuOx.[16][17] Many of the parylenes exhibit this selectivity based on quantum mechanical deactivation of the triplet state, including parylene X. However, like any selective process there is a 'selectivity' window based on mostly deposition pressure and deposition temperature for the parylene polymers. What is more, the intermediate, p-xylylene has a low reactivity and therefore a small 'sticking coefficient' and as a result parylene N produces a highly conformal thin film or coating.
The deposition of parylene N is a function of a two-step process. First, physisorption needs to take place, which is a function of deposition pressure and temperature. The physisorption has inverse Arrhenius kinetics, in other words it is stronger at lower temperatures than higher temperatures. All the parylenes have a critical temperature called the threshold temperature above which practically no deposition is observed. The closer the deposition temperature is to the threshold temperature the weaker the physisorption. Once physisorption occurs, the p-xylylene intermediate needs to react with itself (2nd step) for polymerization to occur. For parylene N, its threshold temperature is 40 °C.
Common halogenated parylenes
Parylene N can be derivatized with respect to its main-chain phenyl ring and its aliphatic carbon bonds. The most common parylene is parylene C (one chlorine group per repeat unit, as shown above) followed by parylene D (two chlorine groups per repeat unit); both chlorine groups are on the main-chain phenyl ring. Because of its higher molecular weight parylene C has a higher threshold temperature, 90 °C, and therefore has a much higher deposition rate, while still possessing a high degree of conformality. It can be deposited at room temperature while still possessing a high degree of conformality and uniformity and a moderate deposition rate >1 nm/s in a batch process. As a moisture diffusion barrier, the efficacy of coatings scales non-linearly with their density. Halogen atoms such as F, Cl and Br add much density to the coating and therefore allow the coating to be a better diffusion barrier. In that regard parylene D is a better diffusion barrier compared to parylene C; however, parylene D suffers from poor across-the-chamber uniformity and conformality at room temperature due to its high molecular weight (135 °C threshold temperature), as a result it is used much less than parylene C.
There are a couple of fluorinated parylenes commercially available, parylene AF-4 (generic name, aliphatic fluorination 4 atoms) [parylene SF (AF-4, Kisco product), parylene HT (AF-4, SCS product)] and parylene VT-4 (generic name, fluorine atoms on the aromatic ring) [also Parylene CF (VT-4, Kisco product)]. Parylene AF-4 is very expensive due to its inefficient wet chemical synthesis of its precursor and its inefficient deposition due to its low polarizability. Polarizability ultimately determines how strongly the intermediate chemistry interacts with the surface and polarizability strongly correlates with molecular weight of the intermediate except in the case of the fluorinated chemistries. Parylene AF-4 is a PTFE (Teflon) analogue in the sense that its aliphatic chemistry has the repeat unit -CF2- and as a result has superior oxidative and UV stability. In contrast, parylene VT-4 (sometimes called just parylene F) has the aliphatic -CH2- chemistry and therefore has poor oxidative and UV stability. Parylene AF-4 has been used to protect outdoor LED displays and lighting from water, salt and pollutants successfully.
The standard Gorham process[18] regardless of the cyclophane starting chemistry is shown above for parylene AF-4. The octafluoro[2.2]paracyclophane is generally sublimed below <100 °C via different configurations. The cyclophane is transported to a pyrolysis zone where it is 'cracked' to the p-xylylene intermediate. This temperature is generally 700-750 °C, higher than the temperature (680 °C) used to crack the hydrocarbon cyclophane since the -CF2-CF2- bond is stronger than the -CH2-CH2- bond. This resonance-stabilized intermediate is transported to a room temperature deposition chamber where polymerization is able to occur under low pressure (1–100 mTorr) conditions. The threshold temperature of parylene AF-4 is very close to room temperature (30–35 °C), as a result, its deposition efficiency is poor. Just one company currently sells this 'dimer'.[19]
An alternate route to parylene AF-4 was developed as shown above. The advantage to this process is the low cost of synthesis for the precursor. The precursor is also a liquid and can be delivered by standard methods developed in the Semiconductor Industry, such as with a vaporizer, vaporizer with a bubbler, or a mass-flow controller. Originally the precursor was just thermally cracked[20] to yield the same intermediate as that produced from the cyclophane; however, with the use of catalysts the 'cracking' temperature can be lowered resulting in less char in the pyrolysis zone and a higher quality polymer thin film.[21][22] By either method atomic bromine (bromine containing a free-radical) is given off as a by-product and is easily converted to hydrogen bromide, which has to be properly processed or equipment damage will occur.
Most recently, the concerns over the bromine by-product has been addressed by a unique route to parylene AF-4, where heavy phenoxy leaving groups are utilized.[23] By this method, the phenoxy leaving group are much 'heavier' than the tetrafluoro-p-xylylene monomer and therefore may condense out, while the monomer is transported cleanly to the deposition chamber where parylene AF-4 is deposited. The organic synthesis route is also very scalable as above (brominated route). The starting compound is the also the starting monomer for polyethylene terephthalate. This route holds the promise to decrease the cost of parylene AF-4 and make its use more widespread.
Reactive parylenes

Most parylenes are passivation thin films or coatings. This means they protect the device or part from environmental stresses such as water, chemical attack, or applied field. This is an important property however many applications have the need to bond other materials to parylene, bond parylene-to-parylene, or even immobilize catalysts or enzymes to the parylene surface. Some of the reactive parylenes are parylene A (one amine per repeat unit, Kisco product), parylene AM (one methylene amine group per repeat unit, Kisco product), and parylene X (a reactive hydrocarbon cross-linkable version, not commercially available). Parylene AM is more reactive than A since it is a stronger base. When adjacent to the phenyl ring the amine group, -NH2-, is in resonance stabilization and therefore becomes more acidic and a result less reactive as a base. However, parylene A is much easier to synthesize and hence it costs less. A side-note, parylene A may be used as an anti-oxidant with parylene C to make it more oxidatively stable in 3-5 wt.%.
Among all the parylenes, parylene X is especially unique since it is: 1) cross-linkable (thermally or with UV light); 2) can generate the Cu-acetylide or Ag-acetylide metalorganic intermediates; 3) can undergo 'Click chemistry'; 4) can be used as an adhesive, parylene-to-parylene bonding without any by-products during processing; 5) is amorphous (non-crystalline); and 6) is a hydrocarbon polymer.
Parylenes as molecular layers
The classic molecular layer chemistries are self-assembled monolayers (SAMs). SAMs are long-chain alkyl chains, which interact with surfaces based on sulfur-metal interaction (alkylthiolates)[24] or a sol-gel type reaction with a hydroxylated oxide surface (trichlorosilyl alkyls or trialkoxy alkyls).[25] However, unless the gold or oxide surface is carefully treated and the alkyl chain is long, these SAMs form disordered monolayers, which do not pack well.[26][27] This lack of packing causes issues in, for example, stiction in MEMS devices.[28]
The observation that parylenes could form ordered molecular layers (MLs) came with contact angle measurements, where MLs thicker than 10 Å had an equilibrium contact angle of 80 degrees (same as bulk parylene N) but those thinner had a reduced contact angle.[29] This was also confirmed with electrical measurements (bias-temperature stress measurements) using metal-insulator-semiconductor capacitors (MISCAPs).[30] In short, parylene N and AF-4 (those parylenes with no functional groups) are pin-hole free at ~14 Å. This results because the parylene repeat units possess a phenyl ring and due to the high electronic polarizability of the phenyl ring adjacent repeat units order themselves in the XY-plane. As a result of this interaction parylene MLs are surface independent, except for transition metals, which de-activate the triplet (benzoid) state and therefore the parylenes cannot be initiated. This finding of parylenes as molecular layers is very powerful for industrial applications because of the robustness of the process and that the MLs are deposited at room temperature. In this way parylenes can be used as diffusion barriers and for reducing the polarizability of surface (de-activation of oxide surfaces). Combining the properties of the reactive parylenes with the observation that they can form dense pin-hole-free molecular layers, parylene X has been utilized as a genome sequencing interface layer.
One caveat with the molecular layer parylenes, namely they are deposited as oligomers and not high polymer.[31] As a result, a vacuum anneal is needed to convert the oligomers to high polymer. For parylene N that temperature is 250 °C, whereas it is 300 °C for payrlene AF-4.
Adhesion
The majority of parylene used is deposited as passivation coatings to passivate the part or device towards moisture, chemical attack or as a dielectric insulator. This in turn often means parylene is coated over complex topographies with many different surface chemistries. If one considers a solid-state material, those materials have three fundamental surfaces when exposed to ambient conditions: 1) noble metal surfaces, 2) metal-oxide forming surfaces, and 3) organic surfaces, e.g. polymeric.
Polymeric surfaces generally only possess dispersion forces but may contain functional groups able to bond to adhesion promoters. If parylene is bonded to a printed circuit board (PCB) then often the mechanical tie-points allow parylene to exhibit good adhesion as opposed to bonding through covalent links (chemical bonding). Sometimes plasma methods are effective in the promotion of adhesion between parylene and polymeric surfaces but these techniques are not trivial to employ. The third surface, metal-oxide forming surfaces, generally possess a hydroxyl-terminated surface, M-OH, where M is a metal such as aluminum or chromium. This termination group has the ability to react with commercially available silanes such as A-174 (methacryloxypropyltrimethoxysilane), which is the common adhesion promoter for the parylene polymers.[32]
The A-174 silane can be vapor delivered in situ or bonded via wet chemical baths. In all cases one half of the molecule binds to metal oxide forming surface through sol-gel chemistry (hydrolysis and condensation) and the other half co-polymerizes with parylene via a free radical addition reaction. In all cases the A-174 silane molecule 'lies down' on the surface and forms self-limited molecular layers of less than 1.0 nm. If thick layers are observed then the silane bath has started to 'polymerize' and a new bath should be started. Vapor phase silylation never yields more than a sub-monolayer of silane on the part being coated; and therefore this problem is circumvented.
History
Parylene development started in 1947, when Michael Szwarc discovered the polymer as one of the thermal decomposition products of a common solvent p-xylene at a temperatures exceeding 1000 °C. Szwarc first postulated the monomer to be para-xylylene, which he confirmed by reacting the vapors with iodine and observing the para-xylylene di-iodide as the only product. The reaction yield was only a few percent, and a more efficient route was found later by William F. Gorham at Union Carbide. He deposited parylene films by the thermal decomposition of [2.2] paracyclophane at temperatures exceeding 550 °C and in vacuum below 1 Torr. This process did not require a solvent and resulted in chemically resistant films free from pinholes.[33][34] Since the coating process takes place at ambient temperature in a mild vacuum, and because of parylene’s conformal properties, it has a wide variety of applications. Union Carbide commercialized a parylene coating system in 1965. Union Carbide went on to undertake research into the synthesis of numerous parylene precursors, including parylene AF-4, throughout the 1960s into the early 1970s. Union Carbide purchased NovaTran (a parylene coater) in 1984 and combined it with other electronic chemical coating businesses to form the Specialty Coating Systems division. The division was sold to Cookson Electronics in 1994.[35] Today there are several parylene coating service companies located around the world.
Characteristics and advantages
- Hydrophobic, chemically resistant coating with good barrier properties for inorganic and organic media, strong acids, caustic solutions, gases and water vapor
- Low leakage current and a low dielectric constant (average in-plane and out-of-plane: 2.67 parylene N and 2.5 parylene AF-4, SF, HT)[36]
- A biostable, biocompatible coating; FDA approved for various applications
- Dense pinhole free, with thickness above 1.4 nm[37]
- Coating without temperature load of the substrates as coating takes place at ambient temperature in a vacuum
- Highly corrosion resistant
- Completely homogeneous surface
- Stable to oxidation up to 350 °C (Parylene AF-4, SF, HT)
- Low intrinsic thin film stress due to its room temperature deposition
- Low coefficient of friction (AF-4, HT, SF)
- Very low permeability to gases
Typical applications
Parylene films have been used in various applications, including[33]
- Hydrophobic coating (moisture barriers, e.g., for biomedical hoses)
- Barrier layers (e.g., for filter, diaphragms, valves)
- Microwave electronics
- Implanted medical devices
- Sensors in rough environment (e.g., automotive fuel/air sensors)
- Electronics for space travel and military
- Corrosion protection for metallic surfaces
- Reinforcement of micro-structures
- Protection of plastic, rubber, etc., from harmful environmental conditions
- Reduction of friction, e.g., for guiding catheters, acupuncture needles and microelectromechanical systems.
References
- ↑ J. B. Fortin & T.-M. Lu (2000). "Mass spectrometry study during the vapor deposition of poly-para-xylylene thin films". Journal of Vacuum Science and Technology A. 18 (5): 2459. doi:10.1116/1.1289773.
- ↑ P. K. Wu, G. -R. Yang, L. You, D. Mathur, A. Cocoziello, C. -I. Lang, J. A. Moore, T. -M. Lu and H. Bakru (1997). "Deposition of High Purity Parylene- F Using Low Pressure Low Temperature Chemical Vapor Deposition". J. Electron Mater. 26 (8): 949–953. Bibcode:1997JEMat..26..949W. doi:10.1007/s11664-997-0280-8.
- ↑ J.J. Senkevich “Non-Halogen Liquid Precursor Route to Parylene” Chem. Vapor Dep. 17(4-6) 76-9 (2011). DOI: 10.1002/cvde.201104304
- ↑ James A. Schwarz; Cristian I. Contescu; Karol Putyera (2004). Dekker encyclopédia of nanoscience and nanotechnology, Volume 1. CRC Press. p. 263. ISBN 978-0-8247-5047-3.
- ↑ C. Chiang, A. S. Mack, C. Pan, Y.-L. Ling, D. B. Fraser Mat. Res. Soc. Symp. Proc. vol. 381, 123 (1995).
- ↑ J. J. Senkevich; B. W. Woods; J. J. McMahon; P.-I Wang (2007). "Thermomechanical Properties of Parylene X, A Room-Temperature Chemical Vapor Depositable Crosslinkable Polymer". Chem. Vapor Dep. 13 (1): 55–59. doi:10.1002/cvde.200606541.
- ↑ J.B. Fortin & T.-M. Lu (2001). "Ultraviolet radiation induced degradation of poly-para-xylylene (parylene) thin films". Thin Solid Films. 397: 223–228. Bibcode:2001TSF...397..223F. doi:10.1016/S0040-6090(01)01355-4.
- ↑ J. J. Senkevich; C. J. Mitchell; A. Vijayaraghavan; E. V. Barnat; J. F. McDonald; T.-M. Lu (2002). "The Unique Structure/Properties of Chemical Vapor Deposited Parylene E". Journal of Vacuum Science and Technology A. 20 (4): 1445–9. doi:10.1116/1.1487870.
- ↑ J.J. Senkevich, “t-butylethynyl-parylene and phenylethynyl-parylene” Chem. Vapor Dep. 19, 1-5 (2013). DOI: 10.1002/cvde.201307071
- ↑ J. F. Gaynor; J. J. Senkevich; S. B. Desu (1996). "A New Method for Fabricating High Performance Polymeric Thin Films by Chemical Vapor Polymerization". J. Mater. Research. 11 (7): 1842–50. Bibcode:1996JMatR..11.1842G. doi:10.1557/JMR.1996.0233.
- ↑ J.J. Senkevich; S. B. Desu (1999). "Near-Room-Temperature Thermal Chemical Vapor Deposition of Poly(chloro-p-xylylene)/SiO2 Nanocomposites". Chemistry of Materials. 11 (7): 1814–21. doi:10.1021/cm990042q.
- ↑ J. J. Senkevich (1999). "CVD of NanoPorous Silica". Chem. Vap. Deposition. 5 (6): 257–60. doi:10.1002/(SICI)1521-3862(199912)5:6<257::AID-CVDE257>3.0.CO;2-J.
- ↑ H. E. Winberg and F. S. Fawcett (1973). "Tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene". Org. Synth.
- ↑ H. J. Reich; D. J. Cram (1969). "Macro rings. XXXVI. Ring expansion, racemization, and isomer interconversions in the [2.2]paracyclophane system through a diradical intermediate". Journal of the American Chemical Society. 91 (13): 3517–3526. doi:10.1021/ja01041a016.
- ↑ P. Kramer; A. K. Sharma; E. E. Hennecke; H. Yasuda (2003). "Polymerization of para-xylylene derivatives (parylene polymerization). I. Deposition kinetics for parylene N and parylene C". Journal of Polymer Science: Polymer Chemistry Edition. 22 (2): 475–491. Bibcode:1984JPoSA..22..475K. doi:10.1002/pol.1984.170220218.
- ↑ K. M. Vaeth & K. F. Jensen (1999). "Selective growth of poly(p-phenylene vinylene) prepared by chemical vapor deposition". Adv. Materials. 11 (10): 814–820. doi:10.1002/(SICI)1521-4095(199907)11:10<814::AID-ADMA814>3.0.CO;2-Z.
- ↑ J.J. Senkevich; C.J. Wiegand; G.-R. Yang; T.-M. Lu (2004). "Selective Deposition of Ultra-thin Poly(p-xylylene) Films on Dielectrics versus Copper Surfaces". Chem. Vapor. Dep. 10 (5): 247–9. doi:10.1002/cvde.200304179.
- ↑ W. F. Gorham (1966). "A New, General Synthetic Method for the Preparation of Linear Poly-p-xylylenes". J. Polym. Sci. A. 4 (12): 3027. Bibcode:1966JPoSA...4.3027G. doi:10.1002/pol.1966.150041209.
- ↑ http://www.gtop-tech-materials.com/
- ↑ Pebalk, A. V.; Kardash, I. E.; Kozlova, N. V.; Zaitseva, E. L.; Kozlov, Yu. A.; Pravednikov, A. N. (1980). Vysokomolekulyarnye Soedineniya, Seriya A. 22 (5): 972–6. Missing or empty
|title=(help) - ↑ Lee, Chung J.; Wang, Hui; Foggiato, Giovanni Antonio, U.S. Patent 6,140,456, Issue date: October 31, 2000.
- ↑ Lee, Chung J., U.S. Patent 6,703,462, Issue date: March 9, 2004.
- ↑ J.J. Senkevich, “Parylene AF-4 via the Trapping of a Phenoxy Leaving Group” Chem. Vapor Dep. 19, 1-5 (2013) DOI: 10.1002/cvde.201304321
- ↑ Laibinis, Paul E.; Whitesides, George M.; Allara, David L.; Tao, Yu Tai; Parikh, Atul N.; Nuzzo, Ralph G. (1991). "Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold". Journal of the American Chemical Society. 113 (19): 7152. doi:10.1021/ja00019a011.
- ↑ Wasserman, Stephen R.; Tao, Yu Tai; Whitesides, George M. (1989). "Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates". Langmuir. 5 (4): 1074. doi:10.1021/la00088a035.
- ↑ Fadeev, Alexander Y.; McCarthy, Thomas J. (2000). "Self-Assembly is Not the Only Reaction Possible between Alkyltrichlorosilanes and Surfaces: Monomolecular and Oligomeric Covalently Attached Layers of Dichloro- and Trichloroalkylsilanes on Silicon". Langmuir. 16 (18): 7268. doi:10.1021/la000471z.
- ↑ Senkevich, Jay J.; Mitchell, Christopher J.; Yang, G.-R.; Lu, T.-M. (2002). "Surface Chemistry of Mercaptan and Growth of Pyridine Short-Chain Alkoxy Silane Molecular Layers". Langmuir. 18 (5): 1587. doi:10.1021/la010970f.
- ↑ Z. Yapu (2003). "Stiction and anti-stiction in MEMS and NEMS". Acta Mechanica Sinica. 19 (1): 1. Bibcode:2003AcMSn..19....1Y. doi:10.1007/BF02487448.
- ↑ Senkevich, Jay J.; Wang, Pei-I (2009). "Molecular Layer Chemistry via Parylenes". Chemical Vapor Deposition. 15 (4–6): 91. doi:10.1002/cvde.200804266.
- ↑ Senkevich, Jay J.; Wang, Pei-I.; Wiegand, Chris J.; Lu, T.-M. (2004). "Bias-Temperature Stability of Ultra Thin Parylene Capped PETEOS Dielectrics: Influence of Surface Oxygen on Copper Ion Diffusion". Applied Physics Letters. 84 (14): 2617. Bibcode:2004ApPhL..84.2617S. doi:10.1063/1.1691488.
- ↑ ^ Senkevich, Jay J.; Wang, Pei-I (2009). "Molecular Layer Chemistry via Parylenes". Chemical Vapor Deposition 15 (4–6): 91. doi:10.1002/cvde.200804266.
- ↑ C. J. Mitchell; G.-R. Yang; J.J. Senkevich (2006). "Adhesion aspects of gamma-methacryloxypropyltrimethoxysilane to poly(p-xylylene)". J. Adhesion Sci. Technol. 20 (14): 1637–1647. doi:10.1163/156856106778884217.
- 1 2 Jeffrey B. Fortin; Toh-Ming Lu (2003). Chemical vapor deposition polymerization: the growth and properties of parylene thin films. Springer. pp. 4–7. ISBN 978-1-4020-7688-6.
- ↑ Mattox, D. M. The foundations of vacuum coating technology, Springer, 2003 ISBN 978-3-540-20410-7 Google books
- ↑ SCS Coatings History. Scscoatings.com. Retrieved on 2012-06-04.
- ↑ J. J. Senkevich; S. B. Desu (1999). "Compositional studies of near-room temperature thermal CVD of poly(chloro-p-xylylene)/SiO2 nanocomposites". Chemistry of Materials. 11 (5): 1814. Bibcode:2000ApPhA..70..541S. doi:10.1007/s003390051076.
- ↑ J. J. Senkevich & P.-I. Wang (2009). "Molecular Layer Chemistry via Parylenes". Chem. Vapor Dep. 15 (4–6): 91. doi:10.1002/cvde.200804266.



