Mitochondrial trifunctional protein
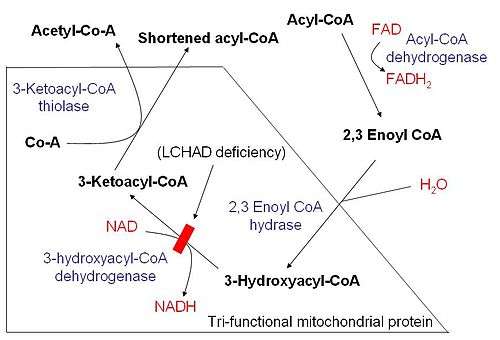
Mitochondrial trifunctional protein (MTP) is a protein attached to the inner mitochondrial membrane which catalyzes three out of the four steps in beta oxidation. MTP is hetero-octamer composed of four alpha and four beta subunits:
The three functions are 2-enoyl coenzyme A (CoA) hydratase, long-chain 3-hydroxy acyl-coenzyme A dehydrogenase and long-chain 3-ketoacyl CoA thiolase.[1]
Association with the Electron Transport Chain
Fatty acid beta-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are two major metabolism pathways in the mitochondria. Reducing equivalents from FAO enter OXPHOS at the level of Complexes I and III. In 2010, Wang et al. discovered a functional and physical association between MTP and ETC respirasomes. Not only does MTP appear to be bound to Complex I, but it also appears to channel substrates between the two enzymes.[2] This is especially interesting, because up until then it was unknown exactly how MTP was associated with the inner mitochondrial membrane, and this discovery may provide the explanation.
Hormone Interaction
Recent research has revealed that MTP can be affected by various hormones, via hormone receptors located in the mitochondria. Chochron et al. (2012) demonstrated that thyroid hormone stimulates mitochondrial metabolism in a pathway mediated by MTP.[3] Zhou et al. (2012) used 2D gel electrophoresis and mass spectrometry to identify MTP as one of the proteins that interacts with ER alpha, a receptor triggered by estrogen.[4]
Cardiolipin Remodeling
In 2009, Taylor et al. identified a human mitochondrial protein, monolysocardiolipin acyltransferase (MLCL AT-1), that is identical in amino acid sequence to the 59-kDa C-terminal end of MTP, linking MTP to the remodeling of cardiolipin from monolysocardiolipin.[5] Although MLCL AT-1 and MTP are different proteins, in 2012 the same lab discovered that MTP did indeed have cardiolipin remodeling capabilities.[6] This suggests a possible link between mitochondrial membrane cardiolipin content and beta oxidation.
Pathology
Disorders are associated with:
References
- ↑ "Long-Chain Acyl CoA Dehydrogenase Deficiency: eMedicine Pediatrics: Genetics and Metabolic Disease". Retrieved 2009-07-11.
- ↑ Wang, Y.; Mohsen, A.-W.; Mihalik, S. J.; Goetzman, E. S.; Vockley, J. (2010). "Evidence for Physical Association of Mitochondrial Fatty Acid Oxidation and Oxidative Phosphorylation Complexes". Journal of Biological Chemistry. 285 (39): 29834–29841. doi:10.1074/jbc.M110.139493. ISSN 0021-9258. PMC 2943265
 . PMID 20663895.
. PMID 20663895. - ↑ Chocron, E. S.; Sayre, N. L.; Holstein, D.; Saelim, N.; Ibdah, J. A.; Dong, L. Q.; Zhu, X.; Cheng, S.-Y.; Lechleiter, J. D. (2012). "The Trifunctional Protein Mediates Thyroid Hormone Receptor-Dependent Stimulation of Mitochondria Metabolism". Molecular Endocrinology. 26 (7): 1117–1128. doi:10.1210/me.2011-1348. ISSN 0888-8809. PMC 3385793
 . PMID 22570332.
. PMID 22570332. - ↑ Zhou, Z.; Zhou, J.; Du, Y. (2012). "Estrogen Receptor Alpha Interacts with Mitochondrial Protein HADHB and Affects Beta-Oxidation Activity". Molecular & Cellular Proteomics. 11 (7): M111.011056–M111.011056. doi:10.1074/mcp.M111.011056. ISSN 1535-9476. PMID 22375075.
- ↑ Taylor, W. A.; Hatch, G. M. (2009). "Identification of the Human Mitochondrial Linoleoyl-coenzyme A Monolysocardiolipin Acyltransferase (MLCL AT-1)". Journal of Biological Chemistry. 284 (44): 30360–30371. doi:10.1074/jbc.M109.048322. ISSN 0021-9258. PMC 2781591
 . PMID 19737925.
. PMID 19737925. - ↑ Beh, Christopher; Taylor, William A.; Mejia, Edgard M.; Mitchell, Ryan W.; Choy, Patrick C.; Sparagna, Genevieve C.; Hatch, Grant M. (2012). "Human Trifunctional Protein Alpha Links Cardiolipin Remodeling to Beta-Oxidation". PLoS ONE. 7 (11): e48628. doi:10.1371/journal.pone.0048628. ISSN 1932-6203. PMC 3494688
 . PMID 23152787.
. PMID 23152787.
External links
- mitochondrial trifunctional protein TP at the US National Library of Medicine Medical Subject Headings (MeSH)