Dimethyldiborane
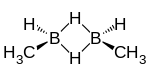 cis-1,2-Dimethyldiborane | |
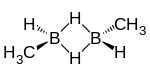 trans-1,2-Dimethyldiborane | |
| Names | |
|---|---|
| IUPAC name
1,2-Dimethyldiborane | |
| Other names
symmetrical dimethyldiborane | |
| Identifiers | |
| 17156-88-6 | |
| 3D model (Jmol) | Interactive image |
| | |
| |
| Properties | |
| (CH3BH2)2 | |
| Molar mass | 55.72 g mol−1 |
| Appearance | Colorless gas |
| Melting point | −124.9 °C (−192.8 °F; 148.2 K) |
| Boiling point | +4 °C (39 °F; 277 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
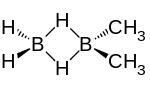 | |
| Names | |
|---|---|
| IUPAC name
1,1-Dimethyldiborane | |
| Other names
unsymmetrical dimethyldiborane | |
| Identifiers | |
| 16924-32-6[1] 7-55-1 | |
| 3D model (Jmol) | Interactive image |
| | |
| |
| Properties | |
| (CH 3) 2B 2H 4 | |
| Molar mass | 55.72 g mol−1 |
| Appearance | Colorless gas |
| Melting point | −423.3 °C; −730.0 °F; −150.2 K |
| Boiling point | −4 °C (25 °F; 269 K) |
| ether pentane tetrahydrofuran | |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
-25 kcal/mol |
| Hazards | |
| NFPA 704 | |
| Related compounds | |
| methyldiborane trimethyldiborane tetramethyldiborane trimethylborane diethyldiborane | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dimethyldiborane is a chemical compound consisting of methyl groups attached to a diborane core. It can be considered as the dimer of methylborane, CH3BH2, the simplest alkylborane, consisting of a methyl group substituted for a hydrogen in borane. There are two isomers. The 1,2-dimethyldiborane form (CH3BH2)2 is symmetrical with one methyl on each boron.[3] 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement.[4] The other isomer is 1,1-dimethyldiborane, known as unsymmetrical dimethyldiborane, which has two methyl groups on one boron atom, and only hydrogen on the other. Other combinations of methylation occur on diborane, including methyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane. Tetramethyldiborane is the dimer of dimethylborane. At room temperature the substance is at equilibrium between these forms.[5] The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.[6][7]
Formation
Methylborane is formed when lithium methylborohydride LiCH3BH3 reacts with an acid in tetrahydrofuran.[3]
2 LiCH3BH3 + 2HCl → (CH3BH2)2 + 2H2 + 2 LiCl
Instead of hydrogen chloride, methyl iodide or trimethylsilyl chloride can be used.[8]
Lithium methylborohydride can be made by reacting a methylboronic ester with lithium aluminium hydride.[8]
Methylboranes are also formed by the reaction of diborane and trimethylborane. This reaction produces four different substitution of methyl with hydrogen on diborane. Produced is 1-methyldiborane, 1,1-dimethyldborane, 1,1,2-trimethyldiborane and 1,1,2,2-tetramethyldiborane. By reacting monomethyldiborane with ether, dimethyl ether borine is formed (CH3)2O.BH3 leaving methylborane which rapidly dimerises to 1,2-dimethyldiborane.[5] The reaction is complex. At 0 °C when diborane is in excess, monomethyldiborane is initially produced, coming to a steady but low level, and 1,1-dimethyldiborane level increases over a long time, until all trimethylborane is consumed. Monomethyldiborane ends up at equilibrium with a mixture of diborane and dimethyldiborane. At 0° the equilibrium constant for 2B2H5Me ←→ B2H6 + (BH2Me)2 is around 0.07, so monomethyldiborane will typically be the majority of the mixture, but there will still be a significant amount of diborane and dimethyldiborane present.[9] Monomethyldiborane yield is best with a ratio of 4 of diborane to 1 of trimethylborane.[10] The yield of trimethyldiborane is maximised with ratio of 1 of diborane to 3 of trimethylborane.[10]
Tetramethyl lead can react with diborane in a 1,2-dimethoxyethane solvent at room temperature to make a range of methyl substituted diboranes, ending up at trimethylborane, but including 1,1-dimethyldiborane, and trimethyldiborane. The other outputs of the reaction are hydrogen gas and lead metal.[11]
Other methods to form methyldiboranes include reacting hydrogen with trimethylborane between 80 and 200 °C under pressure, or reacting a metal borohydride with trimethylborane in the presence of hydrogen chloride, aluminium chloride or boron trichloride. If the borohydride is sodium borohydride, then methane is a side product. If the metal is lithium then no methane is produced.[6] dimethylchloroborane and methyldichloroborane are also produced as gaseous products.[6]
When Cp2Zr(CH3)2 reacts with borane dissolved in tetrahydrofuran, a borohydro group inserts into the zirconium carbon bond, and methyl diboranes are produced.[12]
In ether dimethylcalcium reacts with diborane to produce dimethyldiborane and calcium borohydride.
- Ca(CH3)2 + 2B2H6 → Ca(BH4)2 + B2H4(CH3)2.[13]
Properties
Methylborane can dissolve in liquid pentane, ethyl ether, or tetrahydrofuran.[3]
Unsymmetrical 1,1-dimethyldiborane boils at -4 °C, whereas symmetrical 1,2-dimethyldiborane boils at +4 °C.[14]
Cis-1,2-dimethyldiborane melts at -132.5 °C. Trans-1,2-dimethyldiborane melts at -102 °C.[15] The cis-1,2-dimethyldiborane molecule has point group Cs. A trans-1,2-dimethyldiborane molecule has point group C2.[16] Unsymmetrical dimethyldiborane melts at -150.2 °C.[17] Vapour pressure is approximated by Log P = 7.363-(1212/T).[17] The vapour pressure for the symmetrical isomer is given by Log P = 7.523-(1290/T).[17]
1,1-Dimethyldiborane has a dipole moment of 0.87 d.[18] The predicted heat of formation for the liquid is ΔH0f=-31 kcal/mol, and for the gas -25 kcal/mol. Heat of vapourisation was measured at 5.5 kcal/mol.[19]
A gas chromatograph can be used to determine the amounts of the methyl boranes in a mixture. The order they pass through are diborane, monomethyldiborane, trimethylborane, 1,1-dimethyldiborane, 1,2-dimethyldiborane, trimethyldiborane, and last tetramethyldiborane.[20]
The nuclear resonance shift for the bridge hydrogen is 9.55 ppm for the unsymmetrical isomer and 9.73 ppm for the symmetrical isomers, compared to 10.49 for diborane.[21]
Reactions
In most solvents methylborane reacts with alkenes to produce the dialkylmethylborane. R2CH3BH[3]
However, in tetrahydrofuran, methylborane only reacts with one part alkene to make an alkylmethylborane. (RCH3BH)2[3]
At -78.5 °C methyldiborane disproportionates slowly first to diborane and 1,1-dimethyldiborane.[22] In solution methylborane is more stable against disproportionation than dimethylborane.[8]
1,2-dimethyldiborane slowly converts on standing to 1,1-dimethyldiborane.[24]
Trimethyldiborane partially disproportionates over a period of hours at room temperature to yield tetramethyldiborane and 1,2-dimethyldiborane. Over a period of weeks 1,1-dimethyldiborane appears as well.[25]
Methylborane is hydrolyzed in water to methylboronic acid CH3B(OH)2.[5] Symmetrical dimethyldiborane reacts with trimethylamine to yield a solid derivative trimethylamine-methylborane (CH3)3N—BH2CH3.[5]
Methyldiborane spontaneously inflames when exposed to air.[22]
Gentler oxidation of 1,1-dimethyldiborane at 80 °C yields 2,5-dimethyl-1,3,4-trioxadiboralane, a volatile liquid that contains a ring of two boron and three oxygen atoms.[26] An intermediate in this reaction is two molecules of dimethylborylhydroperoxide (CH3)2BOOH. (CAS 41557-62-5)[27] When methyldiborane is oxidised around 150 °C a similar substance methyltrioxadiboralane is produced. At the same time dimethyltrioxadiboralane and trimethylboroxine are also formed, and also hydrocarbons, diborane, hydrogen, and dimethoxyborane (dimethyl methylboronic ester).[26]
When dimethyldiborane is combined with ammonia and heated, B-methyl borazoles are produced. These borazoles can have one, two or three methyl groups substituted on the bron atoms.[28][29]
Methyl cyanide reacts slowly with 1,1-dimethyldiborane at room temperature to form dimeric ethylideneaminodimethylborane (CH3CH=NB(CH3)2)2 and N,N',N"-triethylborazole.[30]
Under normal conditions dimethyldiborane does not react with hydrogen.[31]
Related
Trihydromethylborate [CH3BH3]− is an ion that can form salts with lithium[8] or the lanthanides.[32]
Other alkyl boranes such as two isomers of diethyldiborane can be produced by analogous methods.[33]
Higher boranes also can form dimethyl compounds, including 1,2- 2,2- and 2,4-dimethyltetraborane,[34] 1,2-dimethylpentaborane[35] 2,3-dimethylpentaborane,[36] 4,5-dimethylhexaborane,[37] and 5,6- 6,8- 6,9-dimethyldecaborane.[38]
References
- ↑ Jane E. Macintyre (ed.). Dictionary of Organometallic Compounds. p. 468.
- ↑ Baker, Charles J. (2001). The Fire Fighter's Handbook of Hazardous Materials. Jones & Bartlett Learning. p. 152.
- 1 2 3 4 5 Srebnik, Morris; Cole, Thomas E.; Brown, Herbert C. (January 1987). "Methylborane - a remarkable unhindered monoalkylborane which achieves the controlled sequential hydroboration of representative alkenes". Tetrahedron Letters. 28 (33): 3771–3774. doi:10.1016/s0040-4039(00)96380-9.
- ↑ Low, M. J. D. (1968). "Characteristic Infrared Frequencies of Methyldiboranes". The Journal of Chemical Physics. 48 (5): 2386. doi:10.1063/1.1669454.
- 1 2 3 4 Bell, R. P.; Emeléus, H. J. (1948). "The boron hydrides and related compounds". Quarterly Reviews, Chemical Society. 2 (2): 132. doi:10.1039/QR9480200132.
- 1 2 3 Long, L. H.; Wallbridge, M. G. H. (1965). "646. The chemistry of boron. Part VI. New preparative methods and decomposition studies relating to methyldiboranes". Journal of the Chemical Society (Resumed): 3513. doi:10.1039/JR9650003513. (subscription required)
- ↑ Schlesinger, H. I.; Walker, A. O. (April 1935). "Hydrides of Boron. IV. The Methyl Derivatives of Diborane". Journal of the American Chemical Society. 57 (4): 621–625. doi:10.1021/ja01307a009.
- 1 2 3 4 Brown, Herbert C.; Cole, Thomas E.; Srebnik, Morris; Kim, Kee Won (December 1986). "Hydroboration. 79. Preparation and properties of methylborane and dimethylborane and their characteristics as hydroborating agents. Synthesis of tertiary alcohols containing methyl groups via hydroboration". The Journal of Organic Chemistry. 51 (25): 4925–4930. doi:10.1021/jo00375a031.
- ↑ van Aalten, Lloyd; Seely, G. R.; Oliver, Juhn; Ritter, D. M. (1 June 1961). "Kinetics and Equilibria in the Alkylation of Diborane Preliminary Report" (PDF). Advances in Chemistry. American Chemical Society: 107–114. doi:10.1021/ba-1961-0032.ch012.
- 1 2 Carpenter, J.H.; Jones, W.J.; Jotham, R.W.; Long, L.H. (September 1971). "The Raman spectra of the methyldiboranes—II Monomethyldiborane and trimethyldiborane, and characteristic frequencies of the methyldiboranes". Spectrochimica Acta Part A: Molecular Spectroscopy. 27 (9): 1721–1734. doi:10.1016/0584-8539(71)80227-1.
- ↑ Holliday, A.K.; N. Jessop, G. (November 1967). "The reaction of tetramethyllead with diborane". Journal of Organometallic Chemistry. 10 (2): 291–293. doi:10.1016/s0022-328x(00)93089-4.
- ↑ Marsella, John A.; Caulton, Kenneth G. (May 1982). "Dealkylation of zirconium(IV) by borane: the intimate mechanism of an alkyl transfer reaction". Journal of the American Chemical Society. 104 (9): 2361–2365. doi:10.1021/ja00373a005.
- ↑ James, B. D.; Wallbridge, M. G. H. (1970). "Metal Tetrahydroborates". In Lippard, Stephen J. Progress in Inorganic Chemistry, Volume 11. Wiley. p. 185. ISBN 0471-54081-1.
- ↑ Lamneck, John H , Jr & Kaye, Samuel (4 September 1958). "Thermal reaction of diborane with trimethylborane". National Advisory Committee for Aeronautics.
- ↑ Hedberg, Lise; Hedberg, Kenneth; Kohler, David A.; Ritter, David M.; Schomaker, Verner (May 1980). "Electron-diffraction investigations of the molecular structures of cis- and trans-1,2-dimethyldiborane". Journal of the American Chemical Society. 102 (10): 3430–3434. doi:10.1021/ja00530a021.
- ↑ Moghadam, M. Eslami; Karimi, T.; Farrokhi D . G., M. (2 January 2012). "Full non-rigid group theory and symmetry of some diborane derivatives" (PDF). International Journal of the Physical Sciences. 7 (1): 73–80. doi:10.5897/IJPS11.1646. Retrieved 13 August 2015.
- 1 2 3 Onak, Thomas (1 January 1966). Stone, F. G. A.; West, Robert, eds. Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. Retrieved 14 August 2015.
- ↑ Chiu, C. W.; Burg, A. B.; Beaudet, R. A. (15 March 1983). "Microwave spectrum, dipole moment, barrier to internal rotation of 1,1‐dimethyldiborane". The Journal of Chemical Physics. 78 (6): 3562–3566. doi:10.1063/1.445182.
- ↑ Altschuller, Aubrey P. (4 October 1955). "Calculated Heats of Formation and Combustion of Boron Compounds (Boron, Hydrogen, Carbon, Silicon)" (PDF). NACA Research Memorandum. Cleveland, Ohio: National Advisory Committee for Aeronautics. p. 22. Retrieved 14 August 2015.
- ↑ Seely, G. R.; Oliver, J. P.; Ritter, D. M. (December 1959). "Gas-Liquid Chromatographic Analysis of Mixtures Containing Methyldiboranes". Analytical Chemistry. 31 (12): 1993–1995. doi:10.1021/ac60156a032.
- ↑ Leach, John B.; Ungermann, Charles B.; Onak, Thomas P. (January 1972). "Proton magnetic resonance studies on methyl and chloro substituted diboranes". Journal of Magnetic Resonance (1969). 6 (1): 74–83. doi:10.1016/0022-2364(72)90088-1.
- 1 2 Bunting, Roger K. (22 Sep 2009). "55 1-Methyldiborane". In Duward F. Shriver. Inorganic Syntheses, Volume 19. John Wiley and Sons. pp. 237–238. ISBN 047104542X.
- 1 2 Onak, Thomas (1 January 1966). "Carboranes and Organo-Substituted Boron Hydrides". In Stone, F. G. A.; West, Robert. Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. Retrieved 19 August 2015.
- ↑ Lehmann, Walter J.; Wilson, Charles O.; Shapiro, I. (1960). "Infrared Spectra of Alkyldiboranes. III. 1,2-Dimethyl- and 1,2-Diethyldiboranes". The Journal of Chemical Physics. 33 (2): 590. doi:10.1063/1.1731190.
- ↑ Lehmann, Walter J.; Wilson, Charles O.; Shapiro, I. (1961). "Infrared Spectra of Alkyldiboranes. V. Tri- and Tetramethyl- and Ethyldiboranes". The Journal of Chemical Physics. 34 (3): 783. doi:10.1063/1.1731675.
- 1 2 Barton, Lawrence; Crump, John M.; Wheatley, Jeffrey B. (June 1974). "Trioxadiborolanes from the oxidation of methyldiborane". Journal of Organometallic Chemistry. 72 (1): C1–C3. doi:10.1016/s0022-328x(00)82027-6.
- ↑ Barton, Lawrence; Crump, John M. (November 1973). "Oxidation of 1,1-dimethyldiborane. Gas-phase peroxide intermediates". Inorganic Chemistry. 12 (11): 2506–2510. doi:10.1021/ic50129a003.
- ↑ Sheldon, J. C.; Smith, B. C. (1960). "The borazoles". Quarterly Reviews, Chemical Society. 14 (2): 202. doi:10.1039/QR9601400200.
- ↑ Schlesinger, H. I.; Horvitz, Leo; Burg, A. B. (March 1936). "Hydrides of Boron. VI. The Action of Ammonia on the Methyl Diboranes". Journal of the American Chemical Society. 58 (3): 409–414. doi:10.1021/ja01294a008.
- ↑ Lloyd, J. E. (July 1964). Reactions of nitriles with some organic compounds of boron and aluminium (Thesis). Durham theses, Durham University.
- ↑ Adams, Roy M. (September 1959). "Organoboron Compounds" (PDF). Advances in Chemistry. 23 (Metal-Organic Compounds): 92. doi:10.1021/ba-1959-0023.ch010. Retrieved 17 August 2015.
- ↑ Greatrex, R. (1987). "Chapter 3. Boron". Annual Reports on the Progress of Chemistry, Section A. 84: 49. doi:10.1039/IC9878400041.
- ↑ Mikhailov, B. M. (April 1962). "The Chemistry Of Diborane". Russian Chemical Reviews. 31 (4): 209.
- ↑ Deever, William R.; Ritter, David M. (November 1969). "Methyltetraboranes. I. 2-Methyl and 1,2-, 2,2-, and 2,4-dimethyl derivatives". Inorganic Chemistry. 8 (11): 2461–2467. doi:10.1021/ic50081a043.
- ↑ Addison, C. C.; Davidson, G. (1973). "Elements of Group III". Inorganic Chemistry of the Main-Group Elements. 1: 68. doi:10.1039/9781847556370-00053. (subscription required)
- ↑ Onak, Thomas; Friedman, Lawrence B.; Hartsuck, Jean A.; Lipscomb, William N. (July 1966). "Rearrangement of 1,2- to 2,3-Dimethylpentaborane(9)". Journal of the American Chemical Society. 88 (14): 3439–3440. doi:10.1021/ja00966a051.
- ↑ Shore, S. G. (1975). "Nido and Arachno Boron Hydrides". In Muetterties, Earl L. Boron Hydride Chemistry. Academic Press. p. 150. ISBN 0-12-509650-X.
- ↑ Dunstan, I.; Williams, R. L.; Blay, N. J. (1960). "970. Boron hydride derivatives. Part V. Nucleophilic substitution in decaborane". Journal of the Chemical Society (IV): 5012. doi:10.1039/JR9600005012. Retrieved 19 August 2015.
Extra reading
- Carpenter, J. H.; Jones, W. J.; Jotham, R. W.; Long, L. H. (1968). "Laser-source Raman spectroscopy and the Raman spectra of the methyldiboranes". Chemical Communications (London) (15): 881. doi:10.1039/C19680000881.
- Lehmann, Walter J.; Wilson, Charles O.; Shapiro, I. (1960). "Infrared Spectra of Alkyldiboranes. I. Monomethyldiboranes". The Journal of Chemical Physics. 32 (4): 1088. doi:10.1063/1.1730853.
- Carpenter, J.H.; Jones, W.J.; Jotham, R.W.; Long, L.H. (June 1970). "The Raman spectra of the methyldiboranes—I 1, 1-dimethyldiborane and tetramethyldiborane". Spectrochimica Acta Part A: Molecular Spectroscopy. 26 (6): 1199–1214. doi:10.1016/0584-8539(70)80027-7.
- Jungfleisch, Francis M. (1973). Reactions of Methyl Substituted Diboranes and 2,2-Dimethyltetraborane with Amine Bases (Thesis). Ohio State University. Retrieved 30 July 2015.
- Isadore Shapiro, C. O. Wilson, J. F. Ditter, W. J. Lehmann (1961). Borax to Boranes (PDF). Advances in Chemistry Series. 32. American Chemical Society. pp. 134–136. doi:10.1021/ba-1961-0032.ch014. mass spectroscopy
- Levison, K. A.; Perkins, P. G. (1970). "Methylaluminium compounds I. The Electronic Structure of Some Methylaluminium and Methylboron Hydrides". Theoretica Chimica Acta. 17 (1): 1–14. doi:10.1007/BF00526759. charge distribution and atom location calculations
