Methyl violet
 | |
| Names | |
|---|---|
| IUPAC name
Methyl violet 2B | |
| Other names
Gentian violet B, Methylrosanilinium chloride, | |
| Identifiers | |
| 8004-87-3 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL64894 |
| ChemSpider | 170606 |
| ECHA InfoCard | 100.111.727 |
| PubChem | 196986 |
| |
| |
| Properties | |
| C24H28N3Cl | |
| Appearance | Green to dark-green powder[1] |
| Melting point | 137 °C (279 °F; 410 K) decomposes[1] |
| Soluble in water, ethanol, insoluble in xylene[1] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink. Methyl violet 10B is also known as crystal violet (and many other names) and has medical uses.[2]
Structure
The term methyl violet encompasses three compounds that differ in the number of methyl groups attached to the amine functional group. They are all soluble in water, ethanol, diethylene glycol and dipropylene glycol.
| Name | Methyl Violet 2B | Methyl violet 6B | Methyl violet 10B (Crystal violet) |
|---|---|---|---|
| Structure |  |  | 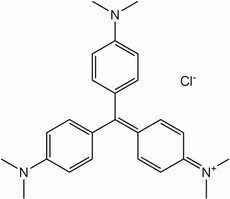 |
| Formula | C23H26N3Cl | C24H28N3Cl | C25H30N3Cl |
| CAS no | 8004-87-3 | 548-62-9 | |
| C.I. | 42535 | 42555 | |
| ChemSpider ID | 21164086 | 170606 | 10588 |
| PubChem ID | 196986 | 11057 | |
| Formula | C23H26N3 | C24H28N3 | C25H30N3 |
| ChemSpider ID | 2006225 | 3349, 9080056, 10354393 | |
| PubChem ID | 2724053 | 3468 |
Methyl violet 2B
Methyl violet 2B (IUPAC name: N-(4-(bis(4-(dimethylamino)phenyl)methylene)cyclohexa-2,5-dien-1-ylidene)methanaminium chloride) is a green powder which is soluble in water in ethanol and water, but not in xylene. It appears yellow in solution of low pH (~0.15) and changes to violet with pH increasing toward 3.2.[1]
Methyl violet 10B
Methyl violet 10B has six methyl groups. It is known in medicine as Gentian violet (or crystal violet or pyoctanin(e)[2]) and is the active ingredient in a Gram stain, used to classify bacteria. It is used as a pH indicator, with a range between 0 and 1.6. The protonated form (found in acidic conditions) is yellow, turning blue-violet above pH levels of 1.6.[3] Gentian violet destroys cells and can be used as a disinfectant.[4] Compounds related to methyl violet are potential carcinogens.
Methyl violet 10B inhibits the growth of many Gram positive bacteria, except streptococci. When used in conjunction with nalidixic acid (which destroys gram-negative bacteria), it can be used to isolate the streptococci bacteria for the diagnosis of an infection.
Degradation
Methyl violet is a mutagen and mitotic poison, therefore concerns exist regarding the ecological impact of the release of methyl violet into the environment. Methyl violet has been used in vast quantities for textile and paper dyeing, and 15% of such dyes produced worldwide are released to environment in wastewater. Numerous methods have been developed to treat methyl violet pollution. The three most prominent are chemical bleaching, biodegradation, and photodegradation.
Chemical bleaching
Chemical bleaching is achieved by oxidation or reduction. Oxidation can destroy the dye completely, e.g. through the use of sodium hypochlorite (NaClO, common bleach) or hydrogen peroxide.[5][6] Reduction of methyl violet occurs in microorganisms but can be attained chemically using sodium dithionite.
Biodegradation
Biodegradation has been well investigated because of its relevance to sewage plants with specialized microorganisms. Two microorganisms that have been studied in depth are the white rot fungus and the bacterium Nocardia Corallina.[7][8]
Photodegradation
Light alone does not rapidly degrade methyl violet,[9] but the process is accelerated upon the addition of large band-gap semiconductors, TiO2 or ZnO.[10][11]
Other methods
Many other methods have been developed to treat the contamination of dyes in a solution, including electrochemical degradation,[12] ion exchange,[13] laser degradation, and absorption onto various solids such as activated charcoal.
See also
References
- 1 2 3 4 R. W. Sabnis (29 March 2010). Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications. John Wiley and Sons. pp. 309–. ISBN 978-0-470-40753-0. Retrieved 27 June 2011.
- 1 2 Gorgas, Ferdinand J. S. (1901). "Pyoctanin – Methyl-Violet – Pyoctanine". chestofbooks.com. Archived from the original on 2011-03-15. Retrieved 2011-03-15.
- ↑ Kristallviolett – ein pH-Indikator Archived June 9, 2011, at the Wayback Machine.
- ↑ WHO Model Lists of Essential Medicines, March 2007
- ↑ Pizzolato, T (2002). "Colour removal with NaClO of dye wastewater from an agate-processing plant in Rio Grande do Sul, Brazil". International Journal of Mineral Processing. 65 (3–4): 203–211. doi:10.1016/S0301-7516(01)00082-5.
- ↑ XP-Chloro Degradation Malachite green U.S. Patent 2,755,202
- ↑ Bumpus, JA; Brock, BJ (1988). "Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium". Applied and Environmental Microbiology. 54 (5): 1143–50. PMC 202618
 . PMID 3389809.
. PMID 3389809.
- ↑ Yatome, Chizuko; Yamada, Shigeyuki; Ogawa, Toshihiko; Matsui, Masaki (1993). "Degradation of Crystal violet by Nocardia corallina". Applied Microbiology and Biotechnology. 38 (4). doi:10.1007/BF00242956.
- ↑ Bhasikuttan, A; Sapre, A.V.; Shastri, L.V. (1995). "Oxidation of crystal violet and malachite green in aqueous solutions — a kinetic spectrophotometric study". Journal of Photochemistry and Photobiology A: Chemistry. 90 (2–3): 177–182. doi:10.1016/1010-6030(95)04094-V.
- ↑ Senthilkumaar, S; Porkodi, K (2005). "Heterogeneous photocatalytic decomposition of Crystal Violet in UV-illuminated sol-gel derived nanocrystalline TiO2 suspensions". Journal of colloid and interface science. 288 (1): 184–9. doi:10.1016/j.jcis.2005.02.066. PMID 15927578.
- ↑ Sahoo, C; Gupta, A; Pal, A (2005). "Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO". Dyes and Pigments. 66 (3): 189–196. doi:10.1016/j.dyepig.2004.09.003.
- ↑ Sanroman, M; Pazos, M; Ricart, M; Cameselle, C (2004). "Electrochemical decolourisation of structurally different dyes". Chemosphere. 57 (3): 233–9. doi:10.1016/j.chemosphere.2004.06.019. PMID 15312740.
- ↑ Wu, J; Liu, C; Chu, K; Suen, S (2008). "Removal of cationic dye methyl violet 2B from water by cation exchange membranes". Journal of Membrane Science. 309: 239–245. doi:10.1016/j.memsci.2007.10.035.