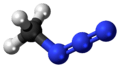Methyl azide
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azidomethane | |||
| Identifiers | |||
| 624-90-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 71411 | ||
| PubChem | 79079 | ||
| |||
| |||
| Properties | |||
| CH3N3 | |||
| Molar mass | 57.05 | ||
| Appearance | white powder | ||
| slightly soluble | |||
| Solubility | alkane, ether | ||
| Explosive data | |||
| Shock sensitivity | High | ||
| Friction sensitivity | High | ||
| Hazards | |||
| Main hazards | Highly explosive | ||
| Related compounds | |||
| Related compounds |
Hydrazoic acid, Chlorine azide, Ethyl azide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
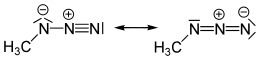
Methyl azide is a covalent molecule related to hydrazoic acid and other alkyl azides.
It can be prepared by a methylation of sodium azide. The first synthesis was reported in 1905.[1] It decomposes in a first-order reaction:[2]
- CH3N3 → CH3N + N2
Methyl azide might be a potential precursor in the synthesis of prebiotic molecules via nonequilibrium reactions on interstellar ices initiated by energetic galactic cosmic rays (GCR) and photons.[3]
Safety precautions
Methyl azide is stable at ambient temperature but may explode when heated.[4] Presence of mercury increases the sensitivity to shock and spark. Incompatible with (dimethyl malonate + sodium methylate); mercury; methanol; sodium azide; dimethyl sulfate; sodium hydroxide; hydrogen azide. When heated to decomposition it emits toxic fumes of NOx.
References
- ↑ Dimroth, O.; Wislicenus, W. (1905). "Ueber das Methylazid". Berichte der Deutschen Chemischen Gesellschaft. 38 (2): 1573–1576. doi:10.1002/cber.19050380254.
- ↑ O'Dell, M. S.; Darwent, B. (1970). "Thermal decomposition of methyl azide". Canadian Journal of Chemistry. 48 (7): 1140–1147. doi:10.1139/v70-187.
- ↑ Quinto-Hernandez, A.; Wodtke, A. M.; Bennett, C. J.; Kim, Y. S.; Kaiser, R. I. (2011). "On the Interaction of Methyl Azide (CH3N3) Ices with Ionizing Radiation: Formation of Methanimine (CH2NH), Hydrogen Cyanide (HCN), and Hydrogen Isocyanide (HNC)". The Journal of Physical Chemistry A. 115 (3): 250–264. doi:10.1021/jp103028v. PMID 21162584.
- ↑ Urben, P. G., ed. (2006). Bretherick's Handbook of Reactive Chemical Hazards (7th ed.). Elsevier. ISBN 9780123725639.
External links
- Graner, G.; Hirota, E.; Iijima, T.; Kuchitsu, K.; Ramsay, D. A.; Vogt, J.; Vogt, N. "CH3N3 Methyl azide". In Kuchitsu, K. Group II Molecules and Radicals: Numerical Data and Functional Relationships in Science and Technology. 25 B. doi:10.1007/10653318_320.
- "Methyl azide". NIST Webbook. National Institute for Standards and Technology.