Iodosobenzene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodosylbenzene | |||
| Other names
Iodosobenzene | |||
| Identifiers | |||
| 536-80-1 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 83171 | ||
| ECHA InfoCard | 100.007.864 | ||
| PubChem | 92125 | ||
| |||
| |||
| Properties | |||
| C6H5IO | |||
| Molar mass | 220.01 g mol−1 | ||
| Appearance | colourless solid | ||
| Density | 1.229 g cm−3 | ||
| Melting point | 210 ˚C | ||
| poor | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula C6H5IO. This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry. It is related to the more popular reagent periodinane.
Preparation and structure
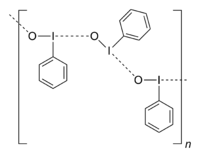
Iodosobenzene was first described by Conrad Willgerodt from iodobenzene.[1] It is prepared by first oxidizing iodobenzene to the diacetate followed by hydrolysis:[2]
The structure of iodosobenzene remains unverified crystallographically. Its low solubility in most solvents and vibrational spectroscopy indicate that it is not molecular, but is polymeric, consisting of I-O-I-O chains.[3] The related diacetate, C6H5I(O2CCH3)2, illustrates the ability of iodine(III) to adopt a T-shaped geometry without multiple bonds.[4] Theoretical studies show that the bonding between the iodine and oxygen atoms in iodosobenzene represents a single dative I-O sigma bond, confirming the absence of the double I=O bond.[5]
Applications
Iodosobenzene has no commercial uses, but in the laboratory it is employed as an "oxo-transfer reagent." It epoxidizes certain alkenes and converts some metal complexes into the corresponding oxo derivatives. Although it is an oxidant, it is also mildly nucleophilic. The active agent in these oxo-transfer reactions is assumed to be monomeric PhI=O, but this aspect remains unverified.
Safety
This compound is explosive and should not be heated under vacuum.
References
- ↑ C. Willgerodt (1892). "Zur Kenntniss aromatischer Jodidchloride, des Jodoso- und Jodobenzols". Ber. 25 (2): 3494–3502. doi:10.1002/cber.189202502221.
- ↑ H. Saltzman and J. G. Sharefkin (1973). "Iodosylbenzene". Org. Synth.; Coll. Vol., 5, p. 658
- ↑ Hans Siebert; Monika Handrich (1976). "Schwingungsspektren und Struktur von Jodosyl- und Jodyl-Verbindungen". Z. anorg. allg. Chem. 426 (2): 173–183. doi:10.1002/zaac.19764260206.
- ↑ C. J. Carmalt; J. G. Crossley; J. G. Knight; P. Lightfoot; A. Martín; M. P. Muldowney; N. C. Norman; A. G. Orpen (1994). "An examination of the structures of iodosylbenzene (PhIO) and the related imido compound, PhINSO2-4-Me-C6H4, by X-ray powder diffraction and EXAFS (extended X-ray absorption fine structure) spectroscopy". J. Chem. Soc., Chem. Commun. (20): 2367. doi:10.1039/C39940002367.
- ↑ Ivanov, A.; Popov, A.; Boldyrev, A.; Zhdankin, V. (2014). "The I=X (X = O,N,C) Double Bond in Hypervalent Iodine Compounds: Is it Real?". Angew. Chem. Int. Ed. doi:10.1002/anie.201405142.