Hydroxylamine-O-sulfonic acid
 | |
 | |
| Identifiers | |
|---|---|
| 2950-43-8 | |
| ECHA InfoCard | 100.019.065 |
| PubChem | 76284 |
| Properties | |
| H3NO4S | |
| Molar mass | 113.09 |
| Appearance | white solid |
| Melting point | 210 °C |
| cold water | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxylamine-O-sulfonic acid ("HOSA") is the inorganic compound with the formula HONHSO3H. It is a white, water-soluble, hygroscopic solid. It is produced formed by the sulfonation of hydroxylamine. It is a reagent for introduction of amino groups, for the conversion of aldehydes into nitriles, alicyclic ketones into lactams and for the formation of variety of nitrogen-containing heterocycles.[1][2][3]
Preparation
According to a laboratory procedure[4] hydroxylamine-O-sulfonic acid can be prepared by treating hydroxylamine sulfate with fuming sulfuric acid (oleum). The industrial process is similar.[5]
![]()
The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid.[6]
Structure
Analogous to sulfamic acid (H3N+SO3-), HOSA exists in the solid state as a zwitterion. It is H3N+OSO3-), consisting of ammonia attached to a sulfate group.[7]
Reactions
HOSA reacts under basic conditions as nucleophile and under neutral and acid conditions as electrophile.[1][8]

Aminations
![]()
It reacts with tertiary amines to trisubstituted hydrazinium salts and with pyridine to the 1-amino pyridinium salt.[9]

From 1-aminopyridinium salts the photochemically active 1-N-iminopyridinium ylides are accessible by acylation.[10] The photochemical rearrangement of the obtained 1-N-iminipyridinium ylides leads in high yields to 1H-1,2-diazepines[11]

N-amination of 1H-benzotriazole with hydroxylamine-O-sulfonic acid yields a mixture of 1-aminobenzotriazole as the main product and 2-aminobenzotriazole.[12] From 1-aminotriazole, dehydrobenzene is formed in an almost quantitative yield by oxidation with lead(IV) acetate, which rapidly dimerizes to biphenylene in good yields.

Electron deficient heterocycles, such as tetrazole, can be N-aminated with hydroxylamine-O-sulfonic acid, while even more electron-deficient compounds, such as 5-nitrotetrazole, react only with stronger aminating agents such as O-tosylhydroxylamine or O- mesitylene sulfonylhydroxylamine to amino compounds, which were investigated as explosives.[13]

In the N-amination of the unsubstituted tetrazole, a mixture of 1-amino- and 2-aminotetrazole is obtained.

Also sulfur compounds (such as thioethers) can be aminated with hydroxylamine-O-sulfonic acid to sulfinimines (isosteric with sulfoxides but far more unstable) or phosphorus compounds (such as triphenylphosphine) can be aminated to phosphinimines via the intermediate aminotriphenylphosphonium hydrogen sulfate.[14]
The reaction of hydroxylamine-O-sulfonic acid with metal salts of sulfinic acids in sodium acetate solution produces primary sulfonamides in very good yields.[15]

Diimine can formed in situ from hydroxylamine-O-sulfonic acid respectively hydroxylamine-O-sulfonic acid hydroxylamine sulfate mixtures, which hydrogenates selectively conjugated multiple bonds. [20]
With carbonyl compounds
At room temperature and below, hydroxylamine-O-sulfonic acid reacts with ketones and aldehydes as a nucleophile to the corresponding oxime-O-sulfonic acids or their salts.[16] The oxime-O-sulfonic acids of aldehydes react above room temperature upon elimination of sulfuric acid in high yields to nitriles.[17]

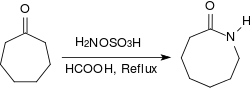
Aliphatic ketones provide under similar conditions in very high yields oximes, arylalkyl ketones react in a Beckmann rearrangement to amides. When refluxed for several hours under acidic conditions (e.g. in the presence of concentrated methanoic acid) alicyclic ketones react to lactams in high yields.[18]
Under basic conditions in the presence of primary amines, hydroxylamine-O-sulfonic acid forms with aldehydes and ketones (eg cyclohexanone[19]) diaziridines, which can easily be oxidized to the more stable diazirines.

The reaction also provides substituted aziridines from simple aldehydes and ketones with high yield and diastereoselectivity.[20]

1,2-benzisoxazole is efficiently produced by nucleophilic attack of hydroxylamine-O-sulfonic acid to the carbonyl group of 2-hydroxybenzaldehyde followed by cyclization.[21]

1,2-Benzisoxazole is a structural element in the antipsychotic risperidone and paliperidone, as well as the anticonvulsant zonisamide.
In a one-pot reaction, N-aryl[3,4-d]pyrazolopyrimidines are obtained in good yields from simple 4,6-dichloropyrimidine-5-carboxaldehyde,[22]

which can be used as purine analogues for a wide range of diagnostic and therapeutic applications.[23]
Further reactions
The chemiluminescence of the system luminol/cobalt(II) chloride is dramatically enhanced by the addition of hydroxylamine-O-sulfonic acid.[24]
References
- 1 2 R.G. Wallace (1980), "Hydroxylamine-O-sulfonic acid – a versatile synthetic reagent", Aldrichimica Acta (in German), 13 (1), pp. 3–11
- ↑ Egon Wiberg; Nils Wiberg (2001). Inorganic Chemistry. Academic Press. pp. 676–7. ISBN 978-0-12-352651-9.
- ↑ Dieter Enders (14 May 2014). Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 40b: Amine N-Oxides, Haloamines, Hydroxylamines and Sulfur Analogues, and Hydrazines. Georg Thieme Verlag. p. 1171. ISBN 978-3-13-172181-5.
- ↑ H.J. Matsuguma; L.F- Audrieth (1957), "Hydroxylamine-O-sulfonic acid" (in German), Inorganic Syntheses 5: pp. 122–125, doi:10.1002/9780470132364.ch32
- ↑ US 0
- ↑ M.W. Rathke; A.A. Millard (1978), "Boranes in functionalization of olefins to amines: 3-Pinanamine" (in German), Org. Synth. 58: pp. 32, doi:10.15227/orgsyn.058.0032
- ↑ Baenziger, Norman C.; Belt, Roger F.; Goebel, Carol V. (1967), "Crystal structure of hydroxylamine-O-sulfonic acid", Inorganic Chemistry, 6 (3): 511–514, doi:10.1021/ic50049a017
- ↑ Erdik, Ender (2001), "Hydroxylamine-O-sulfonic Acid", Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rh058, ISBN 0-471-93623-5
- ↑ R. Gösl; A. Meuwsen (1963), "1-Aminopyridinium iodide" (in German), Org. Synth. 43: pp. 1, doi:10.15227/orgsyn.043.0001
- ↑ J. Streith (1991), "The Photochemistry of N-Iminopyridinium Ylides in Retrospect. From a Simple Concept to Some Applications" (in German), CHIMIA 45 (3): pp. 65–76
- ↑ J. Streith (1977), "The photochemistry of aromatic-N-ylides. Rearrangement and fragmentation patterns" (in German), Pure & Appl. Chem. 49 (3): pp. 305–315, doi:10.1351/pac197749030305
- ↑ C.D. Campbell; C.W. Rees (1969), "Reactive intermediates. Part I. Synthesis and oxidation of 1- and 2-aminobenzotriazole" (in German), J. Chem. Soc. C: pp. 742–747, doi:10.1039/J39690000742
- ↑ T.M. Klapötke; D.G. Piercey; J. Stierstorfer (2012), "Amination of energetic anions: high-performing energetic materials" (in German), Dalton Trans. 41: pp. 9451–9459, doi:10.1039/C2DT30684K
- ↑ R. Appel; W. Büchner; E. Guth (1958), "Zur Kenntnis des Imins, I. Über Phosphinimine und Sulfinimine" (in German), Justus Liebigs Ann. Chem. 618 (1): pp. 53–58, doi:10.1002/jlac.19586180107
- ↑ S.L. Graham; T.H. Scholz (1986), "The reaction of sulfinic acid salts with hydroxylamine-O-sulfonic acid. A useful synthesis of primary sulfonamides" (in German), Synthesis 1986(2): pp. 1031–1032, doi:10.1055/s-1986-31862
- ↑ J. Streith; C. Fizet (1977), "Nucleophilic versus electrophilic properties of the nitrogen atom in O-sulfonyl-hydroxylamine derivatives" (in German), Tetrahedron Lett. 18 (37): pp. 3297–3300, doi:10.1016/S0040-4039(01)83223-8
- ↑ C. Fizet; J. Streith (1974), "Hydroxylamine-o-sulfonic acid: A convenient reagent for the oxidative conversion of aldehydes into nitriles" (in German), Tetrahedron Lett. 15 (36): pp. 3187–3188, doi:10.1016/S0040-4039(01)91857-X
- ↑ G.A. Olah; A.P. Fung (1985), "Hexahydro-2-(1H)-azocinone" (in German), Org. Synth. 63: pp. 188, doi:10.15227/orgsyn.063.0188
- ↑ E. Schmitz; R. Ohme (1965), "3,3-Pentamethylenediaziridine" (in German), Org. Synth. 45: pp. 83, doi:10.15227/orgsyn.045.0083
- ↑ A.W. Beebe; E.F. Dohmeier; G. Moura-Letts (2015), "Diastereoselective synthesis of substituted diaziridines from simple ketones and aldehydes" (in German), Chem. Commun. 51: pp. 13511–13514, doi:10.1039/C5CC04813C
- ↑ D.S. Kemp; R.B. Woodward (1965), "The N-ethylbenzisoxazolium cation—I : Preparation and reactions with nucleophilic species" (in German), Tetrahedron 21 (11): pp. 3019–3035, doi:10.1016/S0040-4020(01)96921-2
- ↑ L.E. Evans; M.D. Cheeseman; K. Jones (2012), "N–N Bond-Forming Cyclization for the One-Pot Synthesis of N-Aryl[3,4-d]pyrazolopyrimidines" (in German), Org. Lett. 14 (13): pp. 3546–3549, doi:10.1021/ol301561a
- ↑ C. Morrill; S. Babu; N.G. Almstead; Y.-C. Moon (2013), "Synthesis of 1,4-disubstituted pyrazolo[3,4-d]pyrimidines from 4,6-dichloropyrimidine-5-carboxaldehyde: insights into selectivity and reactivity" (in German), Synthesis 45: pp. 1791–1806, doi:10.1055/s-0033-1338862
- ↑ M. Saqib; W. Gao; J. Lai; L. Qi; S. Majeed; M.R.H.S. Gilani; G. Xu (2015), "Hydroxylamine-O-sulfonic acid as an efficient coreactant for luminol chemiluminescence for selective and sensitive detection" (in German), Chem. Commun. 51: pp. 6536–6539, doi:10.1039/C5CC01090J