HIST1H2BB
Histone H2B type 1-B is a protein that in humans is encoded by the HIST1H2BB gene.[3][4][5]
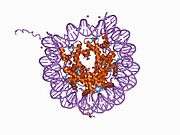
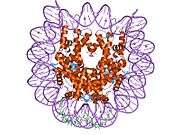
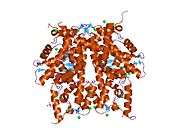
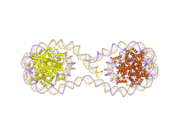
Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Nucleosomes consist of approximately 146 bp of DNA wrapped around a histone octamer composed of pairs of each of the four core histones (H2A, H2B, H3, and H4). The chromatin fiber is further compacted through the interaction of a linker histone, H1, with the DNA between the nucleosomes to form higher order chromatin structures. This gene is intronless and encodes a member of the histone H2B family. Transcripts from this gene lack polyA tails; instead, they contain a palindromic termination element. This gene is found in the large histone gene cluster on chromosome 6p22-p21.3.[5]
References
Further reading
- Albig W, Kardalinou E, Drabent B, et al. (1991). "Isolation and characterization of two human H1 histone genes within clusters of core histone genes". Genomics. 10 (4): 940–8. doi:10.1016/0888-7543(91)90183-F. PMID 1916825.
- Albig W, Kioschis P, Poustka A, et al. (1997). "Human histone gene organization: nonregular arrangement within a large cluster". Genomics. 40 (2): 314–22. doi:10.1006/geno.1996.4592. PMID 9119399.
- Albig W, Doenecke D (1998). "The human histone gene cluster at the D6S105 locus". Hum. Genet. 101 (3): 284–94. doi:10.1007/s004390050630. PMID 9439656.
- El Kharroubi A, Piras G, Zensen R, Martin MA (1998). "Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter". Mol. Cell. Biol. 18 (5): 2535–44. doi:10.1128/mcb.18.5.2535. PMC 110633
 . PMID 9566873.
. PMID 9566873.
- Deng L, de la Fuente C, Fu P, et al. (2001). "Acetylation of HIV-1 Tat by CBP/P300 increases transcription of integrated HIV-1 genome and enhances binding to core histones". Virology. 277 (2): 278–95. doi:10.1006/viro.2000.0593. PMID 11080476.
- Deng L, Wang D, de la Fuente C, et al. (2001). "Enhancement of the p300 HAT activity by HIV-1 Tat on chromatin DNA". Virology. 289 (2): 312–26. doi:10.1006/viro.2001.1129. PMID 11689053.
- Galasinski SC, Louie DF, Gloor KK, et al. (2002). "Global regulation of post-translational modifications on core histones". J. Biol. Chem. 277 (4): 2579–88. doi:10.1074/jbc.M107894200. PMID 11709551.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241
 . PMID 12477932.
. PMID 12477932.
- Cheung WL, Ajiro K, Samejima K, et al. (2003). "Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase". Cell. 113 (4): 507–17. doi:10.1016/S0092-8674(03)00355-6. PMID 12757711.
- Lusic M, Marcello A, Cereseto A, Giacca M (2004). "Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter". EMBO J. 22 (24): 6550–61. doi:10.1093/emboj/cdg631. PMC 291826
 . PMID 14657027.
. PMID 14657027.
- Citterio E, Papait R, Nicassio F, et al. (2004). "Np95 is a histone-binding protein endowed with ubiquitin ligase activity". Mol. Cell. Biol. 24 (6): 2526–35. doi:10.1128/MCB.24.6.2526-2535.2004. PMC 355858
 . PMID 14993289.
. PMID 14993289.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928
 . PMID 15489334.
. PMID 15489334.
- Golebiowski F, Kasprzak KS (2007). "Inhibition of core histones acetylation by carcinogenic nickel(II)". Mol. Cell. Biochem. 279 (1–2): 133–9. doi:10.1007/s11010-005-8285-1. PMID 16283522.
- Zhu B, Zheng Y, Pham AD, et al. (2006). "Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation". Mol. Cell. 20 (4): 601–11. doi:10.1016/j.molcel.2005.09.025. PMID 16307923.
- Bonenfant D, Coulot M, Towbin H, et al. (2006). "Characterization of histone H2A and H2B variants and their post-translational modifications by mass spectrometry". Mol. Cell Proteomics. 5 (3): 541–52. doi:10.1074/mcp.M500288-MCP200. PMID 16319397.
- Pavri R, Zhu B, Li G, et al. (2006). "Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II". Cell. 125 (4): 703–17. doi:10.1016/j.cell.2006.04.029. PMID 16713563.
- Kim SC, Sprung R, Chen Y, et al. (2006). "Substrate and functional diversity of lysine acetylation revealed by a proteomics survey". Mol. Cell. 23 (4): 607–18. doi:10.1016/j.molcel.2006.06.026. PMID 16916647.
PDB gallery |
|---|
|
| 1aoi: COMPLEX BETWEEN NUCLEOSOME CORE PARTICLE (H3,H4,H2A,H2B) AND 146 BP LONG DNA FRAGMENT |
| 1eqz: X-RAY STRUCTURE OF THE NUCLEOSOME CORE PARTICLE AT 2.5 A RESOLUTION |
| 1f66: 2.6 A CRYSTAL STRUCTURE OF A NUCLEOSOME CORE PARTICLE CONTAINING THE VARIANT HISTONE H2A.Z |
| 1hq3: CRYSTAL STRUCTURE OF THE HISTONE-CORE-OCTAMER IN KCL/PHOSPHATE |
| 1kx3: X-Ray Structure of the Nucleosome Core Particle, NCP146, at 2.0 A Resolution |
| 1kx4: X-Ray Structure of the Nucleosome Core Particle, NCP146b, at 2.6 A Resolution |
| 1kx5: X-Ray Structure of the Nucleosome Core Particle, NCP147, at 1.9 A Resolution |
| 1m18: LIGAND BINDING ALTERS THE STRUCTURE AND DYNAMICS OF NUCLEOSOMAL DNA |
| 1m19: LIGAND BINDING ALTERS THE STRUCTURE AND DYNAMICS OF NUCLEOSOMAL DNA |
| 1m1a: LIGAND BINDING ALTERS THE STRUCTURE AND DYNAMICS OF NUCLEOSOMAL DNA |
| 1p34: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3a: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3b: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3f: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3g: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3i: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3k: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3l: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3m: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3o: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1p3p: Crystallographic Studies of Nucleosome Core Particles containing Histone 'Sin' Mutants |
| 1s32: Molecular Recognition of the Nucleosomal 'Supergroove' |
| 1tzy: Crystal Structure of the Core-Histone Octamer to 1.90 Angstrom Resolution |
| 1u35: Crystal structure of the nucleosome core particle containing the histone domain of macroH2A |
| 1zbb: Structure of the 4_601_167 Tetranucleosome |
| 1zla: X-ray Structure of a Kaposi's sarcoma herpesvirus LANA peptide bound to the nucleosomal core |
| 2aro: Crystal Structure Of The Native Histone Octamer To 2.1 Angstrom Resolution, Crystalised In The Presence Of S-Nitrosoglutathione |
| 2cv5: Crystal structure of human nucleosome core particle |
| 2f8n: 2.9 Angstrom X-ray structure of hybrid macroH2A nucleosomes |
| 2fj7: Crystal structure of Nucleosome Core Particle Containing a Poly (dA.dT) Sequence Element |
| 2hio: HISTONE OCTAMER (CHICKEN), CHROMOSOMAL PROTEIN |
| 2nzd: Nucleosome core particle containing 145 bp of DNA |
|
|
 . PMID 9566873.
. PMID 9566873. . PMID 12477932.
. PMID 12477932. . PMID 14657027.
. PMID 14657027. . PMID 14993289.
. PMID 14993289. . PMID 15489334.
. PMID 15489334.