Gramicidin S
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Topical |
| Identifiers | |
| |
| CAS Number |
113-73-5 |
| PubChem (CID) | 73357 |
| ChemSpider |
66085 |
| ChEBI |
CHEBI:5530 |
| ChEMBL |
CHEMBL373496 |
| NIAID ChemDB | 002002 |
| Chemical and physical data | |
| Formula | C60H92N12O10 |
| Molar mass | 1140.7059 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Gramicidin S or Gramicidin Soviet[1] is an antibiotic effective against some Gram positive and Gram negative bacteria as well as some fungi. It is a derivative of gramicidin, produced by the Gram positive bacterium Bacillus brevis. Gramicidin S is a cyclodecapeptide, constructed as two identical pentapeptides joined head to tail, formally written as cyclo(-Val-Orn-Leu-D-Phe-Pro-)2. That is to say, it forms a ring structure composed of five different amino acids, each one used twice within the structure.[2] Another interesting point is that it utilizes two amino acids uncommon in peptides: ornithine as well as the atypical stereoisomer of phenylalanine. It is synthesized by gramicidin S synthetase.[3]
Biosynthesis
Gramicidin S biosynthetic pathway consists of two-enzyme of nonribosomal peptide synthases (NRPSs), gramicidin S synthetase I (GrsA) and gramicidin S synthetase II (GrsB), to give a product as a cyclic decapeptide. Within the biosynthetic pathway, there are total of five modules that specifically recognize, activate, and condense the amino acids to gramicidin S. Starting module GrsA consists of three domains: Adenylation (A) domain where it incorporates the amino acid and activates it by adenylation using ATP, Thiolation (T) domain or peptidyl carrier protein (PCP) in which the adenylated amino acid gets covalently attached to the 4´-phosphopantetheine group and this gets loaded onto the conserved serine in the T domain, Epimerization (E) domain where it epimerizes L-amino acid to D-amino acid. [4] [5] [6] Starting module GrsA loads D-Phe onto the system.
Second enzyme cluster GrsB contains four modules, each containing condensation (C), adenylation (A), and thiolation (T) domains and thioesterase domain (TE) at the end. C domain forms a peptide bond between two amino acids, D-Phe and L-Pro. L-Val, L-Orn, and L-Leu are incorporated sequentially by the next three modules of GrsB. After repeating the whole module synthesis once again, TE domain cyclizes and releases the two peptides and dimerize them together to form the final product. [7]

History
Gramicidin S was discovered by Russian microbiologist Georgyi Frantsevitch Gause and his wife Maria Brazhnikova in 1942. Within the year Gramicidin S was being used in Soviet military hospitals to treat infection and eventually found usage at the front lines of combat by 1946.[8] Gause was awarded the Stalin Prize for Medicine for his discovery in 1946. In 1944, Gramicidin S was sent by the Ministry of Health of the USSR to Great Britain via the International Red Cross in a collaborative effort to establish the exact structure. English chemist Richard Synge proved that the compound was an original antibiotic and a polypeptide using paper chromatography.[9] He would later go on to receive the Nobel Prize for his work in chromatography. The crystal structure was finally established by Dorothy Hodgkin and Gerhard Schmidt; Margaret Thatcher who worked for a term in 1947 with Gerhard Schmidt on the antibiotic Gramicidin S, as an undergraduate research project. The importance of Gramicidin S and antibiotic research in general was so great that Gause was not persecuted during the period of Lysenkoism in the USSR, while many of his colleagues were being executed. Indeed, it was his need for developing new strains to mass-produce antibiotics that allowed politically sanctioned collaborations with geneticists like Joseph Rapoport and Alexander Malinovsky, who would both actively participate in the downfall of Lysenkoism.[8]
Structure and pharmacological effect
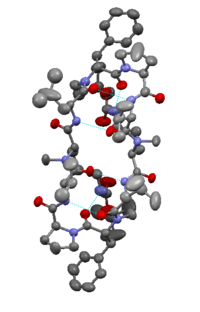
Gramicidin S differs from other gramicidin types in that it is a cationic cyclic decapeptide and has a structure of an anti-parallel beta-sheet. Gramicidin S molecule is amphiphilic, with hydrophobic amino acids (D-Phe, Val, Leu side chains) and charged aminoacid (L-Orn). It exhibits strong antibiotic activity towards Gram-negative and Gram-positive and even several pathogenic fungi. [11] The mode of action is not entirely agreed upon, but it is generally accepted that it is disruption of the lipid membrane and enhancement of the permeability of the bacterial cytoplasmic membrane. [12] Unfortunately, being hemolytic at even low concentrations, gramicidin S is only used as topical applications at present. Additionally, Gramicidin S has been employed as a spermicide and therapeutic for genital ulcers caused by sexually transmitted disease.[13]
References
- ↑ Gause, G. F.; Brazhnikova, M. G. (1944), "Gramicidin S and its use in the Treatment of Infected Wounds", Nature, 154 (3918): 703–703, doi:10.1038/154703a0.
- ↑ Llamas-Saiz, Antonio; Grotenbreg, GM; Overhand, M; Van Raaij, MJ (2007), "Double-stranded helical twisted b-sheet channels in crystals of gramicidin S grown in the presence of trifluoroacetic and hydrochloric acids", Acta Crystallographica Section D, D63 (Pt 3): 401–407, doi:10.1107/S0907444906056435, PMID 17327677
- ↑ Conti, E; Stachelhaus, T; Marahiel, MA; Brick, P (1997), "Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S", The EMBO Journal, 16 (14): 4174–4183, doi:10.1093/emboj/16.14.4174, PMC 1170043
 , PMID 9250661
, PMID 9250661 - ↑ Stachelhaus, T., & Marahiel, M. A. (1995). Modular structure of peptide synthetases revealed by dissection of the multifunctional enzyme GrsA. Journal of Biological Chemistry.
- ↑ Döhren, von, H., Keller, U., Vater, J., & Zocher, R. (1997). Multifunctional Peptide Synthetases. Chemical Reviews, 97(7), 2675–2706.
- ↑ Sieber, S. A., & Marahiel, M. A. (2005). Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chemical Reviews, 105(2), 715–738. http://doi.org/10.1021/cr0301191
- ↑ Miller, L. M., Mazur, M. T., McLoughlin, S. M., & Kelleher, N. L. (2005). Parallel interrogation of covalent intermediates in the biosynthesis of gramicidin S using high-resolution mass spectrometry. Protein Science : a Publication of the Protein Society, 14(10), 2702–2712. http://doi.org/10.1110/ps.051553705
- 1 2 Gall, YM; Konashev, MB (2001), "The discovery of Gramicidin S: the Intellectual Transformation of G.F. Gause from Biologist to Researcher of Antibiotics and on its Meaning for the Fate of Russian Genetics", Hist. Phil. Life Sci., 23 (1): 137–50, PMID 12212443
- ↑ R.L.M. Synge - Britannica Online Encyclopedia
- ↑ Yamada, Keiichi; Unno, Masafumi; Kobayashi, Kyoko; Oku, Hiroyuki; Yamamura, Hatsuo; Araki, Shuki; Matsumoto, Hideyuki; Katakai, Ryoichi; Kawai, Masao (2002), "Stereochemistry of Protected Ornithine Side Chains of Gramicidin S Derivatives: X-ray Crystal Structure of the Bis-Boc-tetra-N-methyl Derivative of Gramicidin S", Journal of the American Chemical Society, 124 (43): 12684–12688, doi:10.1021/ja020307t, PMID 12392415
- ↑ Kondejewski, L. H., Farmer, S. W., Wishart, D. S., Kay, C. M., Hancock, R. E., & Hodges, R. S. (1996). Modulation of structure and antibacterial and hemolytic activity by ring size in cyclic gramicidin S analogs. The Journal of Biological Chemistry, 271(41), 25261–25268.
- ↑ Prenner, E. J., Lewis, R. N., Neuman, K. C., Gruner, S. M., Kondejewski, L. H., Hodges, R. S., & McElhaney, R. N. (1997). Nonlamellar phases induced by the interaction of gramicidin S with lipid bilayers. A possible relationship to membrane-disrupting activity. Biochemistry, 36(25), 7906–7916. http://doi.org/10.1021/bi962785k
- ↑ Krylov YuF (1993) Compendium of medicinal products of Russia. Inpharmchem Press, Moscow, p 343 [in Russian]