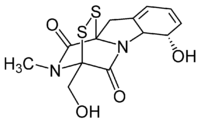
Gliotoxin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3R,6S,10aR)-6-hydroxy-3-(hydroxymethyl)-2-methyl-2,3,6,10-tetrahydro-5aH-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione | |
| Identifiers | |
| 67-99-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL331627 ChEMBL145588 |
| ChemSpider | 5988 |
| ECHA InfoCard | 100.163.992 |
| PubChem | 6223 |
| |
| |
| Properties | |
| C13H14N2O4S2 | |
| Molar mass | 326.4 g/mol |
| Appearance | white to light yellow solid |
| Density | 1.75 g/ml |
| Solubility in DMSO | soluble |
| Hazards | |
| Safety data sheet | MSDS from Fermentek |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines[1] produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics.[2] Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite.
Occurrence
The compound is produced by human pathogens such as Aspergillus fumigatus,[3] and also by species of Trichoderma, and Penicillium. Gliotoxin has also been reported from yeasts of the genus Candida,[4] but results from other studies have cast doubt on the production of this metabolite by Candida fungi.[5][6]
Mechanism of action
Gliotoxin possesses immunosuppressive properties as it may suppress and cause apoptosis in certain types of cells of the immune system, including neutrophils, eosinophils, granulocytes, macrophages, and thymocytes. It also acts as an inhibitor of farnesyl transferase. It noncompetitively inhibits the chymotrypsin-like activity of the 20S proteasome. In vivo it displays anti-inflammatory activity. It acts by blocking thiol groups in the cell membranes. It was investigated as an antibiotic and antifungal in the 1940s and recently as an antiviral agent.[7]
References
- ↑ Borthwick AD (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ↑ Jiang, C.-S.; Muller, W. E. G.; Schroder, H. C.; Guo, Y.-W., "Disulfide- and Multisulfide-Containing Metabolites from Marine Organisms", Chem. Rev. 2012, 112, 2179-2207. doi:10.1021/cr200173z
- ↑ Scharf DH, Heinekamp T, Remme N, Hortschansky P, Brakhage AA, Hertweck C (2012). "Biosynthesis and function of gliotoxin in Aspergillus fumigatus". Appl Microbiol Biotechnol. 93: 467–72. doi:10.1007/s00253-011-3689-1. PMID 22094977.
- ↑ Larsen, B,Shah, D, 1991 "Candida isolates of yeast produce a gliotoxin-like substance" Mycopathologia 116:203-208, 1991.
- ↑ Kupfahl C, Ruppert T, Dietz A, Geginat G, Hof H (2007). "Candida species fail to produce the immunosuppressive secondary metabolite gliotoxin in vitro". FEMS Yeast Res. 7 (6): 986–92. doi:10.1111/j.1567-1364.2007.00256.x. PMID 17537180.
- ↑ Kosalec I, Puel O, Delaforge M, Kopjar N, Antolovic R, Jelic D, Matica B, Galtier P, Pepeljnjak S (2010). "Isolation and cytotoxicity of low-molecular-weight metabolites of Candida albicans". Front Biosci. 13: 6893–904. doi:10.2741/3197. PMID 18508703.
- ↑ McDougall, J. K. (March 2, 2005), "Antiviral action of gliotoxin", Archives of Virology
Further reading
- Identification of an agent in cultures of Aspergillus fumigatus displaying anti-phagocytic and immunomodulating activity in vitro: A. Müllbacher, et al.; J. Gen. Microbiol. 131, 1251 (1985)
- Clinical Isolates of yeast produce a gliotoxin-like substance". D. Shah and B. Larsen; Mycopatholgia 116: 203-208,(1991)
- "Mechanism of gliotoxin action and factors mediating gliotoxin sensitivity". R.W. Jones & J.G. Hancock; J. Gen. Microbiol. 134: 2067-2075 (1988)
- Gliotoxin stimulates Ca2+ release from intact rat liver mitochondria: M. Schweizer & C. Richter; Biochemistry 33, 13401 (1994)
External links
- Puri, A., Ahmad, A. and Panda, B. P. (2010), Development of an HPTLC-based diagnostic method for invasive aspergillosis. Biomed. Chromatogr., 24: 887–892. doi: 10.1002/bmc.1382
- Gliotoxin product page from Fermentek