Germanium disulfide
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Germanium(IV) sulfide[1] | |
| Identifiers | |
| 12025-34-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 74732 |
| ECHA InfoCard | 100.031.537 |
| EC Number | 234-705-1 |
| PubChem | 82816 |
| |
| |
| Properties | |
| GeS2 | |
| Molar mass | 136.75 g·mol−1 |
| Appearance | White, translucent crystals |
| Density | 2.94 g cm−3 |
| Melting point | 840 °C (1,540 °F; 1,110 K) |
| Boiling point | 1,530 °C (2,790 °F; 1,800 K) |
| 0.45 g/100 mL | |
| Solubility | soluble in liquid ammonia |
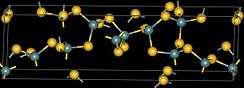
| Structure | |
| monoclinic, mP36 | |
| Pc, No. 7 | |
| tetrahedral at Ge, bent at S | |
| Thermochemistry | |
| 50 J /(mol K) | |
| Std enthalpy of formation (ΔfH |
-150.06 kJ/mol |
| Related compounds | |
| Related compounds |
Carbon disulfide Germanium dioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Germanium disulfide or Germanium(IV) sulfide is the inorganic compound with the formula GeS2. It is a white high-melting crystalline solid.[1][2] The compound is a 3-dimensional polymer, in contrast to silicon disulfide, which is a one-dimensional polymer. The Ge-S distance is 2.19 Å.[3]
History
Germanium disulfide was the first germanium compound found by Clemens Winkler, during the analysis of argyrodite. The fact that germanium sulfide does not dissolve in aqueous acid made it possible for Winkler to isolate the new element.[4]
Production
Germanium disulfide is created by passing hydrogen sulfide with germanium chloride in a concentrated hydrochloric acid solution.
References
- 1 2 Johnson, O. H. (1952). "Germanium and its Inorganic Compounds". Chemical Reviews. 51 (3): 431–469. doi:10.1021/cr60160a002.
- ↑ Golubkov, A. V.; Dubrovskii, G. B.; Shelykh, A. I. (1998). "Preparation and properties of GeS2 single crystals". Semiconductors. 32 (7): 734–735. doi:10.1134/1.1187494.
- ↑ Zachariasen, W. H. (1936). "The Crystal Structure of Germanium Disulphide". Journal of Chemical Physics. 4 (9): 618. doi:10.1063/1.1749915.
- ↑ Winkler, C. (1886). "Mittheilungen über das Germanium". Journal für Praktische Chemie. 34 (1): 177–229. doi:10.1002/prac.18860340122.