Extended porphyrin
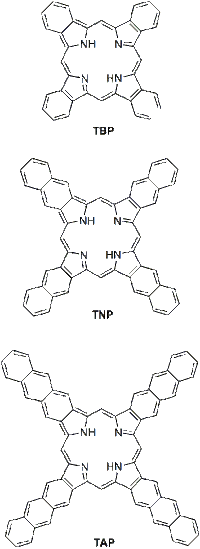
Extented porphyrins, or porphyrins with extended pi-system, form a class of porphyrins in which the pyrrole rings are fused with external aromatic fragments via the β-carbon atoms. The best known and most useful representatives of this class are the linearly annelated π-extended porphyrins, i.e. tetrabenzoporphyrins (TBP), tetranaphthoporphyrins (TNP), tetraanthraporphyrins (TAP), the optical and other properties of which are the subjects of materials research.
Synthesis and structure
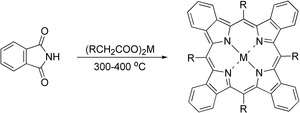
Until recently, the only viable approach to tetrabenzoporphyrins and other extended porphyrins was based on the main synthetic strategy in phthalocyanine chemistry, in which the isoindole residues came from phthalimide or its close analogues being condensed at high temperature in the presence of metal salts acting as templating agents.
Optical properties
Fusion of aromatic fragments to the porphyrin core has a strong and cumulative effect on the energies of excited states of the molecule. It causes a shift of absorption and emission bands into near-infrared region of spectrum.
External links
- Journal of Porphyrins and Phthalocyanines
- Handbook of Porphyrin Science
- Porphynet – an informative site about porphyrins and related structures