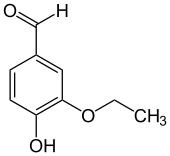
Ethylvanillin
 | |
 | |
| Names | |
|---|---|
| Other names
3-ethoxy-4-hydroxybenzaldehyde, bourbonal | |
| Identifiers | |
| 121-32-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:48408 |
| ChEMBL | ChEMBL508676 |
| ChemSpider | 8154 |
| ECHA InfoCard | 100.004.059 |
| KEGG | D01086 |
| PubChem | 8467 |
| UNII | YC9ST449YJ |
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.18 g·mol−1 |
| Appearance | colourless powder |
| Density | 1.186 g/mL |
| Melting point | 76 °C (169 °F; 349 K) |
| Boiling point | 295.1 °C (563.2 °F; 568.2 K) |
| slightly soluble in water | |
| Hazards | |
| Main hazards | Harmful, Irritant |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ethylvanillin is the organic compound with the formula (C2H5O)(HO)C6H3CHO. This colorless solid consists of a benzene ring with hydroxyl, ethoxy, and formyl groups on the 4, 3, and 1 positions, respectively.
Preparation
Ethylvanillin is a synthetic molecule, not found in nature. It is prepared via several steps from catechol, beginning with ethylation to give "guethol" (1). This ether condenses with glyoxylic acid to give the corresponding mandelic acid derivative (2), which via oxidation (3) and decarboxylation gives ethylvanillin (4).[1]
Application
As a flavorant, ethylvanillin is about three times as potent as vanillin and is used in the production of chocolate.[1]
The molecule revolutionized both the design and aesthetics of olfactory art; artist Aimé Guerlain used it in "Jicky" (1889), one of the earliest uses of the synthetic molecules that freed scent artists from the limits of natural materials.[2]
References
- 1 2 Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim: 2002. Published online: 15 January 2003; doi:10.1002/14356007.a11_141.
- ↑ "Vanillin:Molecule of the Month". Bristol University.
