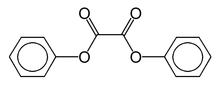
Diphenyl oxalate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
diphenyl oxalate | |
| Other names
diphenylethandioate, oxalic acid diphenyl ester, cyalume, DPO | |
| Identifiers | |
| 3155-16-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 17449 |
| ECHA InfoCard | 100.203.380 |
| PubChem | 18475 |
| |
| |
| Properties | |
| C14H10O4 | |
| Molar mass | 242.227 g/mol |
| Appearance | solid |
| Melting point | 136 °C (277 °F; 409 K) |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
129·0 ± 0·8[1] |
| Related compounds | |
| Related compounds |
Dimethyl oxalate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
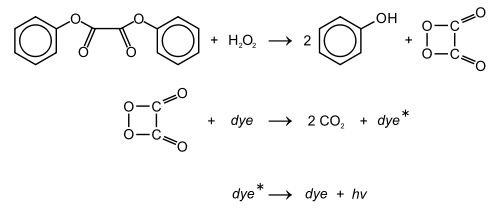
Diphenyl oxalate (trademark name Cyalume) is a solid ester whose oxidation products are responsible for the chemiluminescence in a glowstick. It can be synthesized by fully esterifying phenol with oxalic acid. The reaction with hydrogen peroxide that diphenyl oxalate undergoes produces phenol and 1,2-dioxetanedione,[2] which excites the dye and releases a photon as it decomposes to carbon dioxide.
The reaction rate is pH dependent, and slightly alkaline conditions achieved by adding a weak base, e.g., sodium salicylate, will produce brighter light. The 2,4,6-trichlorophenol ester of oxalic acid is a solid and thus easier to handle. Furthermore, since trichlorophenolate is the better leaving group, the reaction will proceed faster, again producing brighter light, as compared to the phenol ester.
The following colors can be produced by using different dyes:
| Color | Compound |
|---|---|
| Blue | 9,10-Diphenylanthracene |
| Green | 9,10-Bis(phenylethynyl)anthracene |
| Yellow-green | Tetracene |
| Yellow | 1-Chloro-9,10-bis(phenylethynyl)anthracene |
| Orange | 5,12-Bis(phenylethynyl)naphthacene, Rubrene, Rhodamine 6G |
| Red | Rhodamine B |
References
- ↑ Carson, A. S.; Fine, D. H.; Gray, P.; Laye, P. G. (1971). "Standard enthalpies of formation of diphenyl oxalate and benzoic anhydride and some related bond dissociation energies". Journal of the Chemical Society B: Physical Organic: 1611. doi:10.1039/J29710001611.
- ↑ Orosz, György (January 1989). "The role of diaryl oxalates in peroxioxalate chemiluminescence". Tetrahedron. 45 (11): 3493–3506. doi:10.1016/S0040-4020(01)81028-0.