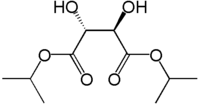
Diisopropyl tartrate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dipropan-2-yl 2,3-dihydroxybutanedioate | |
| Other names
Diisopropyl 2,3-dihydroxysuccinate Diisopropyl tartrate Bis(1-methylethyl) ester of 2,3-dihydroxybutanedioic acid DIPT | |
| Identifiers | |
| 2217-15-4 | |
| 3D model (Jmol) | (−)-isomer: Interactive image Interactive image |
| ChemSpider | 101254 (−)-isomer |
| ECHA InfoCard | 100.017.009 |
| EC Number | 218-709-0 |
| PubChem | 102768 (−)-isomer 112972 |
| |
| |
| Properties | |
| C10H18O6 | |
| Molar mass | 234.25 g/mol |
| Boiling point | 152 °C (306 °F; 425 K) at 16 kPa |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diisopropyl tartrate (DIPT) is a diester of tartaric acid. It has a two chiral carbon atoms giving rise to three stereoisomeric variants. It is commonly used in asymmetric synthesis as a catalyst and as chiral building block for pharmaceuticals and agrochemicals. Its main application is in Sharpless epoxidation, where it serves as a chiral ligand to titanium after reaction with titanium isopropoxide.[1]
References
- ↑ Katsuki, Tsutomu; Sharpless, K. Barry (1980). "The first practical method for asymmetric epoxidation". J. Am. Chem. Soc. 102 (18): 5974. doi:10.1021/ja00538a077.
This article is issued from Wikipedia - version of the 9/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.