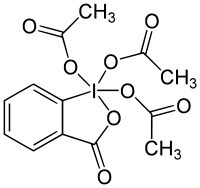
Dess–Martin periodinane
 | |
 | |
| Names | |
|---|---|
| IUPAC names
1,1,1-Triacetoxy-1,1-dihydro- 1,2-benziodoxol-3(1H)-one | |
| Other names
Dess–Martin periodinane | |
| Identifiers | |
| 87413-09-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 139925 |
| ECHA InfoCard | 100.197.885 |
| PubChem | 159087 |
| |
| |
| Properties | |
| C13H13IO8 | |
| Molar mass | 424.14 g/mol |
| Appearance | white powder, chips, crystals or crystalline powder and/or chunks |
| Density | 1.362 g/cm3 solid |
| Melting point | 103 to 133 °C (217 to 271 °F; 376 to 406 K) |
| Related compounds | |
| Related compounds |
2-Iodoxybenzoic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
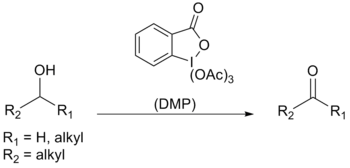
Dess–Martin periodinane (DMP) is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones.[1][2] This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions (room temperature, neutral pH), shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.[3]
Preparation
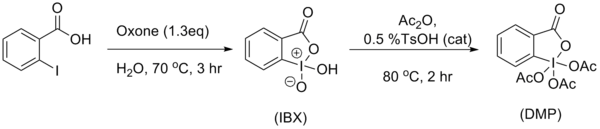
The most friendly synthesis of IBX has been determined to be treating 2-iodobenzoic acid with oxone in water, at elevated temperatures for 3 hours.[4] IBX is then acylated using Ireland and Liu’s [5] modifications from the original procedure. These modifications allowed for higher yields and a simplified work up procedure. The resulted solids can be obtained via filtration and washing with ether. Ireland and Liu used a catalytic amount of tosylic acid, which allowed the reaction to complete in less than 2 hours (compared to the classic synthesis, utilizing 24 hours) and in yields exceeding 90%.
The classic method presented by Boeckman and Mullins[6] involved heating a solution of potassium bromate, sulfuric acid, 2-iodobenzoic acid to afford IBX (1-hydroxy-1,2-benziodoxol-3(1H)-one 1-oxide, 2-iodoxybenzoic acid). IBX was then acylated using acetic acid and acetic anhydride.
Plumb and Harper[7] reported that both 2-iodoxybenzoic acid and DMP were tested to determine their ability to explode, and both samples reacted violently.
Oxidation mechanism
Dess–Martin periodinane is mainly used as an oxidant for complex, sensitive and multifunctional alcohols. One of the reasons for its effectiveness is its high selectivity towards complexation of the hydroxylic group, which allows alcohols to rapidly perform ligand exchange; the first step in the oxidation reaction.
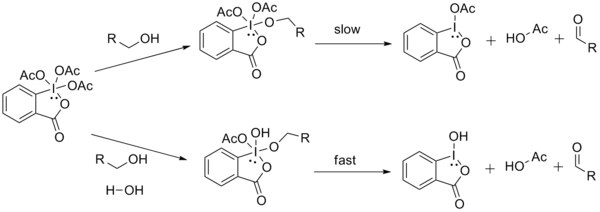
Proton NMR has indicated that using one equivalent of alcohol forms the intermediate diacetoxyalkoxyperiodinane. The acetate then acts as a base to deprotonate the α-H from the alcohol to afford the carbonyl compound, iodinane, and acetic acid.
When a diol or more than one equivalent of alcohol is used, acetoxydialkoxyperiodinane is formed instead. Due to the labile nature of this particular periodinane, oxidation occurs much faster.[3]
Schreiber and coworkers have shown that water increases the rate of the oxidation reaction.[8] Dess and Martin had originally observed that the oxidation of ethanol was increased when there was an extra equivalent of ethanol. It is believed that the rate of dissociation of the final acetate ligand from the iodine is increased, because of the electron-donating ability of the hydroxyl group (thus weakening the I-OAc bond).[3]
Chemoselectivity
Using the standard Dess–Martin periodinane conditions, alcohols can be oxidized to aldehydes/ketones without affecting furan rings, sulfides, vinyl ethers, and secondary amides.[3] Allylic alcohols are easily oxidized using DMP, which are typically difficult to convert to their respective carbonyls using the typical oxidants.[9]
Myers and coworkers determined that DMP could oxidize N-protected-amino alcohols, without epimerization (unlike most other oxidants, including Swern oxidation). These protected amino alcohols can be very important in the pharmaceutical industry.[10]
Benzylic and allylic alcohols react faster than saturated alcohols,[3] while DMP oxidizes aldoximes and ketoximes to their respective aldehydes and ketones, faster than a primary, secondary or benzylic alcohol to its respective carbonyl.[11]
In one example of the Dess–Martin oxidation, involves transforming a sensitive α-β-unsaturated alcohol to its corresponding aldehyde. This moiety has been found in several natural products and due to its high functionality, it could be a valuable synthetic building block in organic synthesis. Thongsornkleeb and Danheiser oxidized this sensitive alcohol by employing the Dess Martin Oxidation and altering the work up procedure (diluting with pentanes, washing with poly(4-vinylpyridine) to remove the acetic acid generated during the reaction, filtering and concentrating via distillation.[12]
t-Butyl DMP
Difluoro and monofluoro alcohols are more difficult to oxidize. Swern oxidation has been used, but a large excess of the oxidant had to be employed, and in some cases did not give reproducible results. Linderman and Graves[13] found DMP was partially successful, but side reactions occurred. Using the modified DM oxidant shown below, produced the wanted carbonyl in high yields. The reason Linderman and Graves chose tert-butyl alcohol as a ligand instead of acetate was due to its bulk and nonoxidizable characteristics. This was to minimize the formation of acetic acid as an unwanted byproduct.
See also
- Swern oxidation
- Parikh–Doering oxidation
- Corey–Kim oxidation
- Pfitzner–Moffatt oxidation
- Pyridinium chlorochromate
- Jones oxidation
- Ley oxidation
- Oppenauer oxidation
References
- ↑ Dess, D. B.; Martin, J. C. (1983). "Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones". J. Org. Chem. 48: 4155. doi:10.1021/jo00170a070.
- ↑ Boeckman, R. J. In "Encyclopedia of Reagents for Organic Synthesis"; Paquette, L. A., Ed.; Wiley: Chichester, UK, 1995, Vol. 7, pp. 4982–4987. (Review)
- 1 2 3 4 5 Dess, D. B.; Martin, J. C. (1991). "A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species". J. Am. Chem. Soc. 113: 7277. doi:10.1021/ja00019a027.
- ↑ Frigerio, M.; Santagostino, M.; Sputore, S. (1999). "A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX)". J. Org. Chem. 64: 4537. doi:10.1021/jo9824596.
- ↑ Ireland, R. E.; Liu, L. (1993). "An improved procedure for the preparation of the Dess-Martin periodinane". J. Org. Chem. 58: 2899. doi:10.1021/jo00062a040.
- ↑ Boeckman Jr., R. K.; Shao, P.; Mullins, J. J. (2004). "The Dess-Martin Periodinane". Org. Synth.; Coll. Vol., 10, p. 696
- ↑ Plumb, J.B.; Harper, D.J. (1990). "Chemical Safety: 2-Iodoxybenzoic acid". Chem. Eng. News. 68: 3. doi:10.1021/cen-v068n029.p002.
- ↑ Meyer, S. D.; Schreiber, S. L. (1994). "Acceleration of the Dess-Martin Oxidation by Water". J. Org. Chem. 59: 7549. doi:10.1021/jo00103a067.
- ↑ Lawrence, N.J.; Crump, J.P.; McGown, A.T.; Hadfield, J.A. (2001). "Reaction of Baylis-Hillman products with Swern and Dess-Martin oxidants.". Tetrahedron Lett. 42: 3939. doi:10.1016/S0040-4039(01)00587-1.
- ↑ Myers, A.G.; et al. (2000). "Synthesis of highly epimerizable N-protected _-amino aldehydes of high enantiomeric excess.". Tetrahedron Lett. 41: 1359. doi:10.1016/S0040-4039(99)02293-5.
- ↑ Chaudhari, S.S.; Akamanchi, K.G. (1999). "A mild, chemoselective, oxidative method for deoximation using Dess-Martin periodinane.". Synthesis. 1999: 760. doi:10.1055/s-1999-3476.
- ↑ Thongsornkleeb, C.; Danheiser, R.L. (2005). "A Practical Method for the Synthesis of 2-Alkynylpropenals". J. Org. Chem. 70: 2364. doi:10.1021/jo047869a.
- ↑ Linderman, R.J.; Graves, D.M. (1989). "Oxidation of Fluoroalkyl-Substituted Carbinols by the Dess-Martin reagent". J. Org. Chem. 54: 661. doi:10.1021/jo00264a029.
External links
| Wikimedia Commons has media related to Dess-Martin periodinane. |