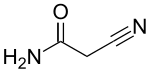
Cyanoacetamide
 | |
| Names | |
|---|---|
| IUPAC name
2-Cyanoacetamide | |
| Other names
Malonamide nitrile 3-Nitrilopropionamide | |
| Identifiers | |
| 107-91-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7610 |
| EC Number | 203-531-8 |
| UNII | YBK38G2YXH |
| |
| |
| Properties | |
| C3H4N2O | |
| Molar mass | 84.08 g·mol−1 |
| Density | 1.163 g/cm3 |
| Melting point | 119 to 121 °C (246 to 250 °F; 392 to 394 K) |
| Boiling point | 351.2 °C (664.2 °F; 624.3 K) |
| Hazards | |
| R-phrases | R8 R23/24/25 R36/38 R45 |
| S-phrases | S17 S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Cyanoacetamide is a organic compound. It is an acetic amide with a nitrile functional group.
Uses
Cyanoacetamide is used in spectrofluorimetric methods to determine the activity of antihistamine H1 receptor antagonistic drugs such as ebastine, cetirizine dihydrochloride and fexofenadine hydrochloride.[1]
Preparation
2-Cyanoacetamide is prepared from chloroacetic acid via Kolbe nitrile synthesis[2] followed by Fischer esterification and ester aminolysis.[3]
See also
References
- ↑ Ibrahim, F.; Sharaf El-Din, M. K.; Eid, M.; Wahba, M. E. K. (2011). "Spectrofluorimetric Determination Of Some H1 Receptor Antagonist Drugs In Pharmaceutical Formulations And Biological Fluids". International Journal of Pharmaceutical Sciences and Research. 21 (8): 2056–2072. doi:10.13040/IJPSR.0975-8232.2(8).2056-72.
- ↑ Inglis, J. K. H. (1928). "Ethyl Cyanoacetate". Organic Syntheses. 8: 74. doi:10.15227/orgsyn.008.0074.
- ↑ Corson, B. B.; Scott, R. W.; Vose, C. E. (1941). "Cyanoacetamide". Organic Syntheses. 1: 179. doi:10.15227/orgsyn.009.0036.
This article is issued from Wikipedia - version of the 10/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.