Corey–House synthesis
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium dialkyl cuprate with an alkyl halide to form a new alkane, an organic copper compound and a lithium halide.[1][2][3]
- R2CuLi + R'-X → R-R' + RCu + LiX
Reaction mechanism
This reaction occurs in three steps. The alkyl halide is treated with lithium metal, and solvated in dry ether, which converts the alkyl halide into an alkyl lithium compound, R-Li. The starting R-X can be primary, secondary or tertiary alkyl halide:
- R-X + 2Li → R-Li + Li-X
The second step requires the alkyl lithium compound to be treated with cuprous iodide (CuI). This creates a lithium dialkyl cuprate compound. These compounds were first synthesized by Henry Gilman of Iowa State University, and are usually called Gilman reagents in honor of his contributions:
- 2RLi + CuI → R2CuLi + LiI
The lithium dialkyl cuprate is then treated with the second alkyl halide, which couples to the compound:
- R2CuLi + R'-X → R-R' + RCu + LiX
If second alkyl halide is not the same as the first, then cross-products are formed.
It is important to note that for this reaction to work successfully, the second alkyl halide must be a methyl halide, benzyl halide, primary alkyl halide or a secondary cyclo alkyl halide. The relative simplicity of this reaction makes it a useful technique for synthesizing organic compounds.
Catalytic version
Kochi demonstrated that Grignard reagents and alkyl bromides could be coupled using a catalytic amount of lithium tetrachlorocuprate(II),[4][5] a process that was extended to alkyl tosylates by Schlosser and Fouquet.[6]
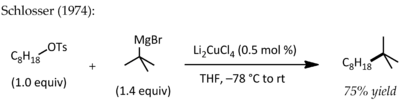
Background
This reaction was developed by four organic chemists (two at Harvard and two at MIT):
E.J. Corey of Harvard University, former mentor of Gary H. Posner (later of Johns Hopkins University), as well as
Herbert O. House of MIT (later of Georgia Tech) and his colleague George M. Whitesides (later of Harvard University)
See also
References
- ↑ Posner, G. H. (1975). "Substitution Reactions using Organo Copper Reagents". Organic Reactions. 22. p. 253. doi:10.1002/0471264180.or022.02.
- ↑ Corey, Elias J.; Posner, Gary H. (19 July 1967). "Selective formation of carbon-carbon bonds between unlike groups using organocopper reagents". Journal of the American Chemical Society. 89 (15): 3911–3912. doi:10.1021/ja00991a049.
- ↑ House, Herbert O.; Respess, William L.; Whitesides, George M. (1 October 1966). "The Chemistry of Carbanions. XII. The Role of Copper in the Conjugate Addition of Organometallic Reagents". The Journal of Organic Chemistry. 31 (10): 3128–3141. doi:10.1021/jo01348a012.
- ↑ TAMURA, M.; KOCHI, J. (1971-01-01). "Coupling of Grignard Reagents with Organic Halides". Synthesis. 1971 (06): 303–305. doi:10.1055/s-1971-35043. ISSN 0039-7881.
- ↑ Kochi, Jay K. (1978). Organometallic Mechanisms and Catalysis. New York: Academic Press. pp. 381–386. ISBN 0-12-418250-X.
- ↑ Fouquet, Gerd; Schlosser, Manfred (1974-01-01). "Improved Carbon-Carbon Linking by Controlled Copper Catalysis". Angewandte Chemie International Edition in English. 13 (1): 82–83. doi:10.1002/anie.197400821. ISSN 1521-3773.