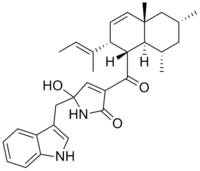
Codinaeopsin
 | |
| Names | |
|---|---|
| IUPAC name
5-((1H-indol-3-yl)methyl)-3-((1R,2R,4aS,6R,8S,8aS)-2-((E)-but-2-en-2-yl)-4a,6,8-trimethyl-1,2,4a,5,6,7,8,8a-octahydronaphthalene-1-carbonyl)-5-hydroxy-1H-pyrrol-2(5'H)-one | |
| Properties | |
| C31H38N2O3 | |
| Molar mass | 486.66 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Codinaeopsin is an antimalarial isolated from a fungal isolate found in white yemeri trees (Vochysia guatemalensis) in Costa Rica. It is reported to have bioactivity against Plasmodium falciparum with an IC50 = 2.3 µg/mL (4.7 µM). Pure codinaeopsin was reported to be isolated with a total yield of 18 mg/mL from cultured fungus.[1] The biosynthesis of codinaeopsin involves a polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid.
Biosynthesis
Formation of linear polyketide
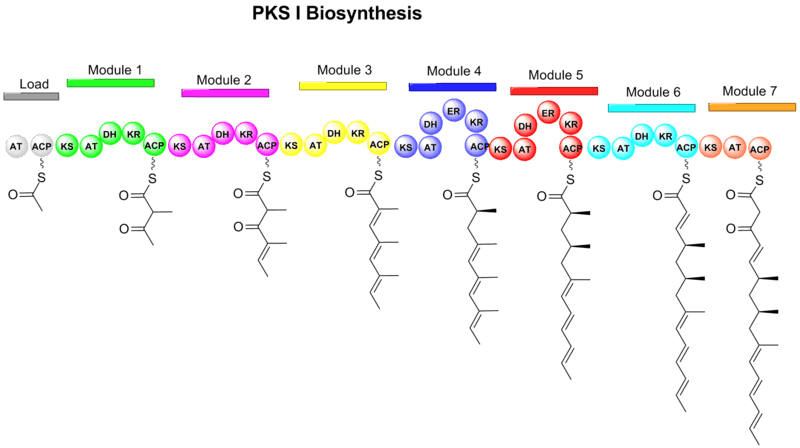
The first step of the biosynthesis of codinaeopsin involves the assembly of the a linear polyketide by use of seven modules and incorporation of six methylmalonyl CoAs and one malonyl CoA by polyketide synthases (type I PKSs).[1]
Formation of tetramic acid (2,4-pyrrolidinone)
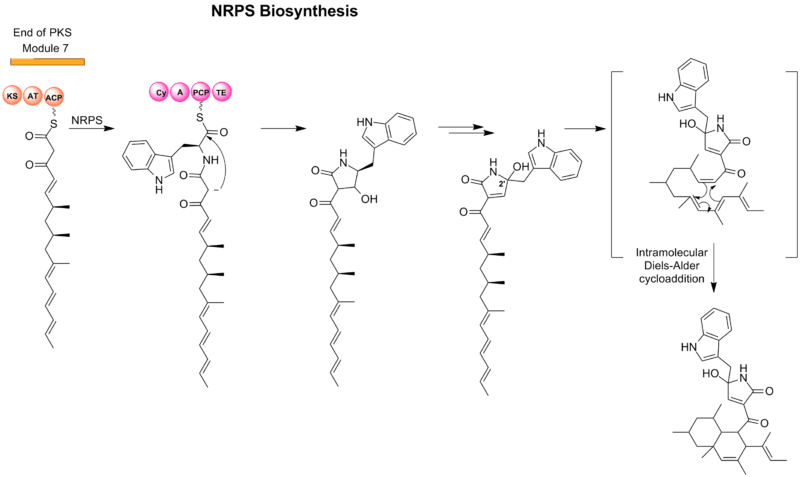
L-Tryptophan is introduced by a nonribosomal peptide synthetase (NRPS) module and results in the central heterocyclic tetramic acid (2,4-pyrrolidinone). The formal oxidation-reduction is found to be achieved by a series of tautomeric shifts involving enol and imine intermediates in the ring and consistent by discovery both C-2’ epimers.[1]
Cyclization of PKS-assembled unit
The PKS unit is hypothesized to cyclize by a Diels-Alder-like addition similar to other natural products such as lovastatin and solanapyrone.[2]
References
- 1 2 3 Kontnik, Renee; Clardy, Jon (2008). "Codinaeopsin, an Antimalarial Fungal Polyketide". Org. Lett. 10 (18): 4149–4151. doi:10.1021/ol801726k.
- ↑ Auclair, Karine; Sutherland, Andrew; Kennedy, Jonathan; Witter, David J.; Van den Heever, Johan P.; Hutchinson, C. Richard; Vederas, John C. (2000). "Lovastatin Nonaketide Synthase Catalyzes an Intramolecular Diels−Alder Reaction of a Substrate Analogue". Journal of the American Chemical Society. 122 (46): 11519–11520. doi:10.1021/ja003216+. ISSN 0002-7863.