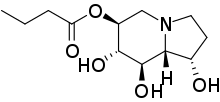
Celgosivir
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 6-O-Butanoylcastanospermine; MDL-28574; MX-3253 |
| PubChem (CID) |
60734 CID 3033824 |
| ChemSpider | 54737 |
| Chemical and physical data | |
| Formula | C12H21NO5 |
| Molar mass | 259.30 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Celgosivir, in development by Migenix for the treatment of hepatitis C virus (HCV) infection, is an oral prodrug of the natural product castanospermine that inhibits alpha-glucosidase I, an enzyme that plays a critical role in viral maturation by initiating the processing of the N-linked oligosaccharides of viral envelope glycoproteins. Celgosivir is well absorbed in vitro and in vivo, and is rapidly converted to castanospermine. Celgosivir has a novel mechanism of action (i.e. host-directed glycosylation), and demonstrates broad antiviral activity in vitro.[1]
Clinical trials
Celgosivir is not efficient as a monotherapy for the treatment of HCV, but has demonstrated a synergistic effect in combination with pegylated interferon alfa-2b plus ribavirin, both in vitro and in phase II clinical trials that last up to 1 year in patients with chronic HCV infection. Celgosivir may prove to be a valuable component for combination therapy and may help to prevent the apparition of drug resistance. Long-term toxicity studies are necessary to confirm the safety of this novel drug in humans.[1]
Although generally safe and well tolerated, celgosivir does not seem to reduce viral load or fever burden in patients with dengue fever.[2]
References
- 1 2 Durantel, D (2009). "Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection". Current opinion in investigational drugs (London, England : 2000). 10 (8): 860–70. PMID 19649930.
- ↑ Low, Jenny G; Sung, Cynthia; Wijaya, Limin; Wei, Yuan; Rathore, Abhay P S; Watanabe, Satoru; Tan, Boon Hian; Toh, Liying; Chua, Lian Tee; Hou, Yan'an; Chow, Angelia; Howe, Shiqin; Chan, Wing Ki; Tan, Kah Hin; Chung, Jasmine S; Cherng, Benjamin P; Lye, David C; Tambayah, Paul A; Ng, Lee Ching; Connolly, John; Hibberd, Martin L; Leo, Yee Sin; Cheung, Yin Bun; Ooi, Eng Eong; Vasudevan, Subhash G (2014). "Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial". The Lancet Infectious Diseases. 14 (8): 706. doi:10.1016/S1473-3099(14)70730-3. PMID 24877997.