Cadiot–Chodkiewicz coupling
| Cadiot–Chodkiewicz coupling | |
|---|---|
| Named after | Paul Cadiot Wladyslav Chodkiewicz |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | cadiot-chodkiewicz-coupling |
| RSC ontology ID | RXNO:0000100 |
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base.[1][2] The reaction product is a 1,3-diyne or di-alkyne.
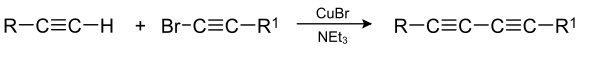
The reaction mechanism involves deprotonation by base of the terminal alkyne proton followed by formation of a copper(I) acetylide. A cycle of oxidative addition and reductive elimination on the copper centre then creates a new carbon-carbon bond.
Related couplings are the Glaser coupling and the Eglinton coupling.
Scope
In one study[3] the Cadiot–Chodkiewicz coupling has been applied in the synthesis of acetylene macrocycles starting from cis-1,4-diethynyl-1,4-dimethoxycyclohexa-2,5-diene. This compound is also the starting material for the dibromide through NBS and silver nitrate:
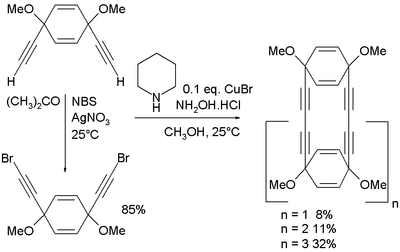
The coupling reaction itself takes place in methanol with piperidine, the hydrochloric acid salt of hydroxylamine and copper(I) bromide.
See also
- Glaser coupling - Another alkyne coupling reaction catalysed by copper (I).
- Sonogashira coupling - Pd/Cu catalysed coupling of an alkyne with an aryl or vinyl halide
- Castro–Stephens coupling - A cross-coupling reaction between a copper(I) acetylide and an aryl halide
References
- ↑ Chodkiewicz, W. Ann. Chim. Paris 1957, 2, 819–69.
- ↑ Cadiot, P.; Chodkiewicz, W. In Chemistry of Acetylenes; Viehe, H. G., Ed.; Marcel Dekker: New York, 1969; pp 597–647.
- ↑ Bandyopadhyay, Arkasish; Varghese, Babu; Sankararaman, Sethuraman. "Synthesis of 1,4-Cyclohexadiene-Based Acetylenic Macrocycles with Cadiot−Chodkiewicz Coupling. Structure of a Tub-Shaped Tetrameric Container". The Journal of Organic Chemistry. 71 (12): 4544–4548. doi:10.1021/jo0605290.