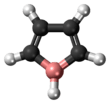
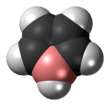
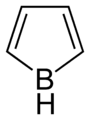
Borole
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1H-Borole | |||
| Identifiers | |||
| 287-87-6 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 10709008 | ||
| |||
| |||
| Properties | |||
| C4H5B | |||
| Molar mass | 63.89 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Borole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with boron replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been isolated. Substituted derivatives, which have been synthesized, are called boroles. Although the Hückel's rule cannot be strictly applied to borole, it is considered to be anti-aromatic.[1]
Reactions
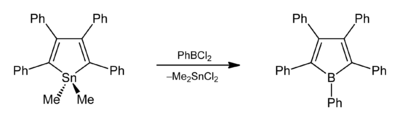
The first borole to be isolated, pentaphenylborole, can be formed by the reaction of 1,1-dimethyl-2,3,4,5-tetraphenylstannole and phenylboron dichloride.[2] Boroles can be used to form ferrocene-like sandwich compounds.[3]
See also
References
- ↑ Alan R. Katritzky, ed. (1993), Advances in Heterocyclic Chemistry, 56, Academic Press, p. 375, ISBN 978-0-12-020756-5, retrieved 2010-03-13
- ↑ Francis Gordon Albert Stone, Robert West, ed. (1996), Advances in Heterocyclic Chemistry, 39, Academic Press, p. 380, ISBN 978-0-12-031139-2, retrieved 2010-03-13
- ↑ Alan R. Katritzky, ed. (2001), Advances in Heterocyclic Chemistry, 79, Academic Press, pp. 169–170, ISBN 978-0-12-020779-4, retrieved 2010-03-13
This article is issued from Wikipedia - version of the 10/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.