Aspergillusol A
 | |
| Names | |
|---|---|
| IUPAC name
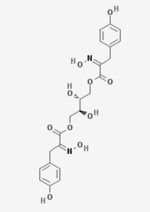
[(2S,3R)-2,3-Dihydroxy-4-[(2Z)-2-hydroxyimino-3-(4-hydroxyphenyl)propanoyl]oxybutyl] (2Z)-2-hydroxyimino-3-(4-hydroxyphenyl)propanoate | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 24667436 |
| PubChem | 44605528 |
| |
| |
| Properties | |
| C22H24N2O10 | |
| Molar mass | 476.44 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aspergillusol A is an alpha-glucosidase inhibitor isolated from marine Aspergillus.[1] Structurally, it consists of an erythritol group in the center, with two hydroxyimino-phenylpropanoyl groups attached to it from either side. It was synthesized in 2010 by researchers from King Fahd University of Petroleum and Minerals, starting with 4-hydroxybenzaldehyde.[2]
References
- ↑ Ingavat, N; Dobereiner, J; Wiyakrutta, S; Mahidol, C; Ruchirawat, S; Kittakoop, P (2009). "Aspergillusol A, an alpha-glucosidase inhibitor from the marine-derived fungus Aspergillus aculeatus". Journal of Natural Products. 72 (11): 2049–52. doi:10.1021/np9003883. PMID 19824618.
- ↑ Ullah, N.; Haladu, S. A. (2010). "The first total synthesis of aspergillusol A, an alpha-glucosidase inhibitor". Natural product communications. 5 (7): 1077–1080. PMID 20734944.
This article is issued from Wikipedia - version of the 1/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.