Alisertib
 | |
| Names | |
|---|---|
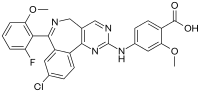
| IUPAC name
4-{[9-Chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}-2-methoxybenzoic acid | |
| Systematic IUPAC name
4-{[9-Chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}-2-methoxybenzoic acid | |
| Identifiers | |
| 1028486-01-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL483158 |
| ChemSpider | 24700147 |
| 7790 | |
| KEGG | D10085 |
| PubChem | 24771867 |
| UNII | T66ES73M18 |
| |
| |
| Properties | |
| C27H20ClFN4O4 | |
| Molar mass | 518.93 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alisertib (MLN8237) is an orally available selective aurora A kinase inhibitor developed by Takeda.[1] It is currently being investigated as a treatment for relapsed or refractory peripheral T-cell lymphoma.[2][3]
References
- ↑ Friedberg, JW; Mahadevan, D; Cebula, E; Persky, D; Lossos, I; Agarwal, AB; Jung, J; Burack, R; Zhou, X; Leonard, EJ; Fingert, H; Danaee, H; Bernstein, SH (Jan 1, 2014). "Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas.". Journal of Clinical Oncology. 32 (1): 44–50. doi:10.1200/JCO.2012.46.8793. PMID 24043741.
- ↑ "Millennium Initiates Pivotal Phase 3 Trial of MLN8237 in Patients With Relapsed or Refractory Peripheral T-cell Lymphoma". Takeda Pharmaceutical Company Limited; Millennium Pharmaceuticals, Inc. March 6, 2012. Retrieved 20 March 2014.
- ↑ "Research and Development Pipeline (As of February 5, 2014)" (PDF). Takeda Pharmaceutical Company Limited. February 5, 2014. p. 2. Retrieved 20 March 2014.
This article is issued from Wikipedia - version of the 7/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.