Achim Müller
| Achim Müller | |
|---|---|
 | |
| Born |
14 February 1938 Detmold, Germany |
| Nationality | German |
| Fields | Chemistry |
| Institutions | University of Bielefeld |
| Alma mater | University of Göttingen |
| Known for | Tailor-made porous nanoclusters and their use as versatile materials |
Achim Müller (born 14 February 1938 in Detmold, Germany) is a German scientist. He is Professor Emeritus at the Faculty of Chemistry, University of Bielefeld.
Academic career
Achim Müller studied chemistry and physics at the University of Göttingen and received there his PhD degree (1965) and the Habilitation (1967). In 1971, he became professor at the University of Dortmund and in 1977 professor of inorganic chemistry at the University of Bielefeld. His research involves the chemistry of transition metals in synthesis, spectroscopy, and theory especially with relation to nanochemistry,[1][2] bioinorganic chemistry including biological nitrogen fixation,[3] molecular magnets (even one showing quantum oscillations),[4][5] molecular physics (e.g. theoretical and experimental investigations of the isotope influence on molecular constants) as well as history and philosophy of science.[6] He has also published about 900 original papers in more than 100 different journals related to different fields, more than 40 reviews and is coeditor of 16 books (see External links below). Achim Müller is a member of several national, e.g. the Leopoldina[7] and international academies, e.g. Polish Academy of Sciences, The Indian National Science Academy, National Academy of Exact Physical and Natural Sciences (Argentina), Academia Europaea and has received many awards (honorary doctor degrees, -professorships and -memberships; see External links) and prizes (e.g. Alfred Stock Memorial Prize 2000, Prix Gay-Lussac/Humboldt 2001, Sir Geoffrey Wilkinson Prize 2001, Centenary Medal of the Royal Society of Chemistry 2008/9, London,[8] and named lectureships. In 2012 he was awarded with the prestigious Advanced Grant by the European Research Council (ERC) (for more honors see External links). From Nature Chemistry he got an invitation to write an article about the future of inorganic chemistry for the first issue of the journal in 2009 ("Predicting a structured future").
Research
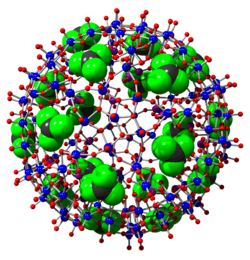
His currently most compelling research relates mainly to bottom-up pathways towards tailor-made porous metal oxide nanoclusters (like Mo132; model purchasable from a company: http://www.moleculart3d.com/) and their use as versatile materials of interdisciplinary character.[9] This includes the following topics (see especially Ref.[1]):
- Processes, also catalytic ones, under confined conditions, especially in capsules with stepwise closable pores and tunable internal functions
- Tuning hydrophobicity of nanocontainer interiors to influence, for example, encapsulated water to allow studying aspects of the hydrophobic effect
- Chemical adaptability in nanomaterials
- Multi-supramolecular chemistry on sphere surfaces
- Modelling cation transport through membranes and their separation in small spaces
- Studying new solution states for inorganic ions through vesicle (blackberry) formation
- Coordination chemistry at surfaces, in pores, and in cavities of nanocapsules
- Encapsulation chemistry in general including related reactions in small spaces
- Versatile linking of nanoclusters in different phases, i.e., to films, monolayers, and in the gas phase
- Examples for a supramolecular-chemical Darwinism
- Recognition of hydrophobic species in water in connection with hydrophobic clustering in porous capsules
- Controlled exchange of guests at different internal porous capsule sites with each other and with the outside
- Porous capsules acting as carriers even for many cations and an ions together
- Modelling ion channels based on surfactant encapsulated porous metal-oxide capsules with hydrophobic interiors
- Repellency of a nanodrop of water from the internal hydrophobic capsule surface
- Encapsulated giant water clusters of high an low density type
- Unprecedented molecule-based magnets
- The beauty of Keplerates
Müller's discovery of the molecular giant spheres (Keplerates) of the type Mo132 (diameter ca. 3 nm) and their derivatives,[10] of the wheel shaped cluster Mo154 (Refs. 1 and[11]) and hedgehog shaped cluster Mo368 (as large as 6 nm) has caused a paradigm shift regarding their sizes and especially due to their unique properties as nanomaterials. These single molecules are quite large; this can be shown by taking the length of an oxygen molecule with two atoms (length 0.12 nm) as a unit, then considering Mo368 which is 50 times larger. Müller's related work shows many applications (see above), for example, how cellular processes like ion-transport can be modeled based on the spherical porous capsules[12][13] and how the latter can be used to remove toxic compounds from water.[14] All the mentioned nanomaterials belong to a class commonly termed polyoxometalates and some special ones to the molybdenum blue family.[15][16] The compounds are studied worldwide by many groups especially related to problems of Materials Science (see Ref. [1]). One aspect is modelling of the Lotus effect,[17] another one chemical adaptability as a new phenomenon.[18] An interesting mathematical treatment of the Keplerates could be developed in relation to spherical viruses and Buckminster Fuller Domes based on Archimedean and Platonic solids.[19] Applications of the Mo132 Keplerate are also reported by several other authors, e.g.:[20] "Thus, Keplerate-type capsules represent unique supramolecular objects offering a tunable spatially-restricted environment and promising in many domains such as catalysis, electric conductivity, non-linear optics, liquid crystals, vesicles and "blackberry" aggregates." (a summary of different publications) "... a key point to promote confined space engineering."
Personal
Müller likes ancient Greek philosophy, classical music, and mountain hiking. He has a love for woodland birds since his early childhood, a pastime which his father cherished also.
References
- ↑ From linking of metal-oxide building blocks in a dynamic library to giant clusters with unique properties and towards adaptive chemistry; a) A. Müller, P. Gouzerh, From linking of metal-oxide building blocks in a dynamic library to giant clusters with unique properties and towards adaptive chemistry, Chem. Soc. Rev., 2012, 41, 7431; b) A. Müller, P. Gouzerh, Capsules with Highly Active Pores and Interiors: Versatile Platforms at the Nanoscale, Chem. Eur. J. (Concept Article) 2014, 20, 4862-4873.
- ↑ Supramolecular Inorganic Chemistry: Small Guests in Small and Large Hosts, A. Müller, H. Reuter, S. Dillinger, Angew. Chem. Int. Ed. Engl.,1995, 34, 1071.
- ↑ a) Iron-only nitrogenase: exceptional catalytic, structural and spectroscopic features, in: Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models, and Commercial Processes, K. Schneider, A. Müller (Eds.: B. E. Smith, R. L. Richards, W. E. Newton), Kluwer, Dordrecht (2004), p. 281; b) Towards Biological Supramolecular Chemistry: A Variety of Pocket-Templated, Individual Metal Oxide Cluster Nucleations in the Cavity of a Mo/W-Storage Protein, J. Schemberg, K. Schneider, U. Demmer, E. Warkentin, A. Müller, U. Ermler, Angew. Chem. Int. Ed., 2007, 46, 2408; corrigendum: 2007, 46, 2970.
- ↑ Structure-related frustrated magnetism of nanosized polyoxometalates: aesthetics and properties in harmony, P. Kögerler, B. Tsukerblat, A. Müller, Dalton Trans., 2010 (perspective article) 39, 21.
- ↑ Quantum oscillations in a molecular magnet, S. Bertaina, S. Gambarelli, T. Mitra, B. Tsukerblat, A. Müller, B. Barbara, Nature, 2008, 453, 203; corrigendum: Nature, 2010, 466, 1006 (V15).
- ↑ For example: a) Die inhärente Potentialität materieller (chemischer) Systeme, A. Müller, Philosophia naturalis, 1998, Bd. 35, Heft 2, 333; b) Naturgesetzlichkeiten – Chemie lediglich ein Bereich zwischen Physik und biologischen Gesetzen ?, A. Müller, Philosophia naturalis, 2000, Bd. 37, Heft 2, 351; c) Chemie und Ästhetik - die Formenvielfalt der Natur als Ausdruck ihrer Kreativität, A. Müller, ZiF (Center for Interdisciplinary Research), Mitteilungen, 1999, 4, 7; d) Science, Society, and Hopes of a Renaissance Utopist, A. Müller, Science & Society, 2000, 1, 23.
- ↑ http://www.leopoldina.org/en/members/list-of-members/member/548/ Nationale Akademie der Wissenschaften Leopoldina
- ↑ http://www.rsc.org/ScienceAndTechnology/Awards/CentenaryPrizes/Prevwinner1.asp
- ↑ Molecular growth from a Mo176 to a Mo248 cluster, A. Müller, S. Q. N. Shah, H. Bögge, M. Schmidtmann, Nature, 1999, 397, 48.
- ↑ Picking up 30 CO2 Molecules by a Porous Metal Oxide Capsule Based on the Same Number of Receptors, S. Garai, E. T. K. Haupt, H. Bögge, A. Merca, A. Müller, Angew. Chem. Int. Ed., 2012, 51, 10528.
- ↑ Self-assembly in aqueous solution of wheel-shaped Mo154 oxide clusters into vesicles, T. Liu, E. Diemann, H. Li, A.W.M. Dress, A. Müller, Nature, 426, 2003, 59.
- ↑ Guests on Different Internal Capsule Sites Exchange with Each Other and with the Outside, O. Petina, D. Rehder, E.T.K. Haupt, A. Grego, I.A. Weinstock, A. Merca, H. Bögge, J. Szakacs, A. Müller, Angew. Chem. Int. Ed. 2011, 50, 410.
- ↑ Mimicking Biological Cation-Transport Based on Sphere-Surface Supramolecular Chemistry: Simultaneous Interaction of Porous Capsules with Molecular Plugs and Passing Cations, A. Merca, E.T.K. Haupt, T. Mitra, H. Bögge, D. Rehder, A. Müller, Chem. Eur. J., 2007, 13, 7650.
- ↑ a) Hydrophobic Interactions and Clustering in a Porous Capsule: Option to Remove Hydrophobic Materials from Water, C. Schäffer, A. M. Todea, H. Bögge, O. A. Petina, D. Rehder, E.T.K. Haupt, A. Müller, Chem. Eur. J.,2011, 17, 9634; b) Densely Packed Hydrophobic Clustering: Encapsulated Valerates Form a High-Temperature-Stable {Mo132} Capsule System, S. Garai, H. Bögge, A. Merca, O. A. Petina, A. Grego, P. Gouzerh, E. T. K. Haupt, I. A. Weinstock, A. Müller, Angew. Chem. Int. Ed., 2016, 55, 6634 (front cover picture).
- ↑ A Nanosized Molybdenum Oxide Wheel with a Unique Electronic-Necklace Structure: STM Study with Submolecular Resolution, D. Zhong, F. L. Sousa, A. Müller, L. Chi, H. Fuchs, Angew. Chem. Int. Ed., 2011, 50, 7018.
- ↑ Soluble Molybdenum blue-"des Pudels Kern", A. Müller, C. Serain, Acc. Chem. Res., 2000, 33, 2.
- ↑ Water Repellency in Hydrophobic Nanocapsules - Molecular View on Dewetting, A. Müller, S. Garai, C. Schäffer, A. Merca, H. Bögge, A. J. M. Al-Karawi, T. K. Prasad, Chem. Eur. J. 2014, 20, 6659 (cover picture).
- ↑ Chemical Adaptability: The Integration of Different Kinds of Matter into Giant Molecular Metal Oxides, A. Müller, A. Merca, A. J. M. Al-Karawi, S. Garai, H. Bögge, G. Hou, L. Wu, E. T. K. Haupt, D. Rehder, F. Haso, T. Liu, Chem. Eur. J., 2012, 18, 16310.
- ↑ a) Spherical (Icosahedral) Objects in Nature and Deliberately Constructable Molecular Keplerates: Structural and Topological Aspects, O. Delgado, A. Dress, A. Müller, in: Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, M. T. Pope, A. Müller Eds., Kluwer, Dordrecht, 2001; b) A chemist finds beauty in molecules that resemble an early model of the Solar System, A. Müller, Nature 2007, 447, 1035; c) The Beauty of Symmetry, A. Müller, Science 2003, 300, 749.
- ↑ Tunable Keplerate Type-Cluster "Mo132" Cavity with Dicarboxylate Anions, T.-L. Lai, M. Awada, S. Floquet, C. Roch-Marchal, N. Watfa, J. Marrot, M. Haouas, F. Tauelle, E. Cadot, Chem. Eur. J., 2015, 21, 13311.
Bibliography
- Honorary issue of Inorganica Chimica Acta (Biography)
- From Scheele and Berzelius to Müller: polyoxometalates (POMs) revisited and the "missing link" between the bottom up and top down approaches, P. Gouzerh, M. Che, l’actualité chimique, 2006, June Issue, No. 298, 9.
- Inorganic Molecular Capsules: From Structure to Function, L. Cronin, Angew. Chem. Int. Ed., 2006, 45, 3576.
- Bringing inorganic chemistry to life, N. Hall, Chem. Commun., 2003, 803 (focus article).
- Author Profile, Angew. Chem. Int. Ed., 2013, 52, 800.