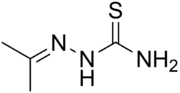
Acetone thiosemicarbazone
 | |
| Names | |
|---|---|
| IUPAC name
(Propan-2-ylideneamino)thiourea | |
| Other names
Acetone thiosemicarbazide; Dimethyl ketone thiosemicarbazone; Thiosemicarbazone acetone; NSC 711; 2-(1-Methylethylidene)hydrazinecarbothioamide | |
| Identifiers | |
| 1752-30-3 | |
| 3D model (Jmol) | Interactive image Interactive image |
| Abbreviations | ATSC |
| ChEMBL | ChEMBL500557 |
| ChemSpider | 2050659 |
| ECHA InfoCard | 100.015.580 |
| EC Number | 217-137-9 |
| PubChem | 2770166 |
| |
| |
| Properties | |
| C4H9N3S | |
| Molar mass | 131.20 g·mol−1 |
| Appearance | White crystals |
| Melting point | 172 to 175 °C (342 to 347 °F; 445 to 448 K) |
| Hazards | |
| Main hazards | Toxic |
| Safety data sheet | MSDS |
| EU classification (DSD) |
T+ |
| R-phrases | R21 R25 R26 |
| S-phrases | S22 S28 S36/37/39 S45 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acetone thiosemicarbazone is a chemical compound with the molecular formula C4H9N3S. It is used in the plastics industry in the manufacture of polyvinyl chloride (PVC) to terminate the polymerization process.[2][3]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[4]
References
- ↑ Acetone thiosemicarbazone, chemicalland21.com
- ↑ US patent 3637632, Traynor, Lee, "Agents for shortstopping free radical polymerization of vinylidene monomers", issued 1972-Jan-25
- ↑ Acetone thiosemicarbazone (ATSC) Archived February 27, 2012, at the Wayback Machine., 88chem.com
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
This article is issued from Wikipedia - version of the 11/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
