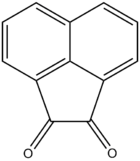
Acenaphthoquinone
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acenaphthylene-1,2-dione | |
| Other names
Acenaphthoquinone (no longer accepted even in general nomenclature[1]) Acenaphthenequinone 1,2-Acenaphthenequinone Acenaphthenedione 1,2-Acenaphthylenedione Acenaphthene-1,2-dione 1,2-Diketoacenaphthene | |
| Identifiers | |
| 82-86-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15342 |
| ChEMBL | ChEMBL395653 |
| ChemSpider | 6468 |
| ECHA InfoCard | 100.001.311 |
| EC Number | 201-441-3 |
| KEGG | C02807 |
| PubChem | 6724 |
| |
| |
| Properties | |
| C12H6O2 | |
| Molar mass | 182.18 g·mol−1 |
| Appearance | Purple-yellow crystals to brown powder |
| Melting point | 257 to 261 °C (495 to 502 °F; 530 to 534 K) |
| Insoluble (90.1 mg/l) | |
| Hazards | |
| Main hazards | Irritating |
| R-phrases | R36/37/38 |
| S-phrases | S26, S37/39 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acenaphthoquinone is a quinone derived from acenaphthene. It is insoluble in water, but soluble in alcohol. It is used as an intermediate for the manufacturing of dyes, pharmaceuticals and pesticides. It is also used in chemical research as a drug and therapeutic agent.
The substance is classified as an irritant. Its carcinogenic properties have not been fully investigated yet.
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 724. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
External links
- National Pollutant Inventory - Polycyclic Aromatic Hydrocarbon Fact Sheet
- PAHs - including structural diagrams
- Entry at ChemicalLand21.com
- MSDS at chemphys.gcsu.edu
This article is issued from Wikipedia - version of the 12/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
