5α-Androst-2-ene-17-one
Not to be confused with Androstenone.
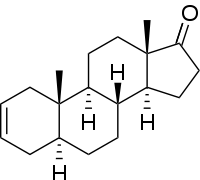 | |
| Names | |
|---|---|
| IUPAC name
(5S,8R,9S,10S,13S,14S)-10,13-Dimethyl-1,4,5,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
17-Oxo-5α-androst-2-ene; Delta-2-androst-17-one; (+)-Androst-2-en-17-one | |
| Identifiers | |
| 963-75-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 92089 |
| ECHA InfoCard | 100.012.289 |
| PubChem | 239425 |
| |
| |
| Properties | |
| C19H28O | |
| Molar mass | 272.43 g·mol−1 |
| Melting point | 106 to 107 °C (223 to 225 °F; 379 to 380 K)[1] |
| Pharmacology | |
| |
| Oral | |
| Pharmacokinetics: | |
| Liver | |
| Urine | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5α-Androst-2-ene-17-one is a steroid. It is referred to commercially as delta-2-androst-17-one. This compound is a naturally occurring pheromone found in elephants and boars.[2] 5α-Androst-2-ene-17-one is also a natural human metabolite of DHEA.[3][4]
References
- ↑ Bockova, H. (1966). Collection of Czechoslovak Chemical Communications. 31 (9): P3790–3799. doi:10.1135/cccc19663790.
- ↑ Marchlewska-Koj, A.; Lepri, J. J. (2001). "Chemical signals in vertebrates 9". Springer. 9: 131. ISBN 978-0-306-46682-3.
- ↑ Callies, F; Arlt, W; Siekmann, L; Hübler, D; Bidlingmaier, F; Allolio, B (2000). "Influence of oral dehydroepiandrosterone (DHEA) on urinary steroid metabolites in males and females.". Steroids. 65 (2): 98–102. doi:10.1016/S0039-128X(99)00090-2. PMID 10639021.
- ↑ Gower, DB; Mallet, AI; Watkins, WJ; Wallace, LM (1997). "Transformations of steroid sulphates by human axillary bacteria. A mechanism for human odour formation?". Biochemical Society Transactions. 25 (1): 16S. doi:10.1042/bst025016s. PMID 9056914.
This article is issued from Wikipedia - version of the 2/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.