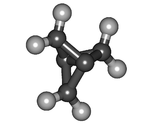
1.1.1-Propellane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tricyclo[1.1.1.01,3]pentane | |||
| Identifiers | |||
| 35634-10-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 125285 | ||
| PubChem | 142022 | ||
| |||
| |||
| Properties | |||
| C5H6 | |||
| Molar mass | 66.10 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(>CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
[1.1.1]Propellane is a highly strained molecule. The bonds of the two central carbon atoms have an inverted tetrahedral geometry, and the length of the central bond is 160 pm. The strength of that bond is disputed; estimates vary from 59–65 kcal/mol to no strength at all. The energy of the biradical state (without the central bond and unfilled central carbons) is calculated to be 80 kcal/mol higher. The compound is highly unstable, and at 114 °C it will spontaneously isomerize to 3-methylidenecyclobutene with a half-life of 5 minutes. Its strain energy is estimated to be 102 kcal/mol (427 kJ/mol).
The type of bonding in this molecule has been explained in terms of charge-shift bonding.[1]
Synthesis
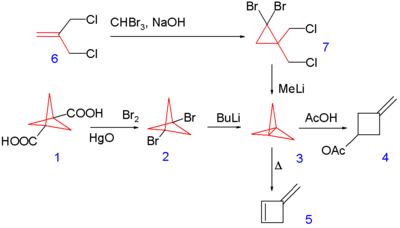
[1.1.1]Propellane was first synthesized by K. Wiberg and F. Walker in 1982,[2] according to the following scheme:
 Scheme 1. Synthesis of [1.1.1]propellane
Scheme 1. Synthesis of [1.1.1]propellane
Synthesis begins with conversion of the 1,3-di-carboxylic acid of bicyclo[1.1.1]pentane 1 in a Hunsdiecker reaction to the corresponding dibromide 2 followed by a coupling reaction with n-butyllithium. The final product 3 was isolated by column chromatography at −30 °C.
However, a much simplified synthesis was published by Szeimies.[3] It starts with dibromocarbene addition to the alkene bond of 3-chloro-2-(chloromethyl)propene 6 followed by deprotonation by methyllithium and nucleophilic displacements in 7[4] not isolated but kept in solution at −196 °C.
Reactions
Acetic acid addition
[1.1.1]Propellane spontaneously reacts with acetic acid to yield a methylidenecyclobutane ester (4 above).
Polymerization
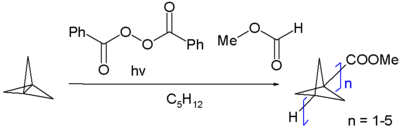
[1.1.1]Propellane undergoes a polymerization reaction where the central C–C bond is split and connected to adjacent monomer units, resulting the so-called staffanes.[5]
 Scheme 2. Synthesis of [n]staffane.
Scheme 2. Synthesis of [n]staffane.
A radical polymerization initiated by methyl formate and benzoyl peroxide results in a distribution of oligomers. An anionic addition polymerization with n-butyllithium results in a fully polymerized product. X-ray diffraction of the polymer shows that the connecting C–C bonds have bond lengths of only 148 pm.
The compound 1,3-dehydroadamantane, which can be viewed as a bridged [1.3.3]propellane, also polymerizes in a similar way.
See also
- [2.2.2]Propellane
- [3.3.3]Propellane
References
- ↑ Wu, Wei; Gu, Junjing; Song, Jinshuai; Shaik, Sason; Hiberty, Philippe C. (2009). "The Inverted Bond in [1.1.1]Propellane is a Charge-Shift Bond". Angew. Chem. Int. Ed. 48: 1407–1410. doi:10.1002/anie.200804965.
- ↑ Wiberg, K. B.; Walker, F. H. (1982). "[1.1.1]Propellane". J. Am. Chem. Soc. 104 (19): 5239–5240. doi:10.1021/ja00383a046.
- ↑ Belzner, Johannes; Bunz, Uwe; Semmler, Klaus; Szeimies, Günter; Opitz, Klaus; Schlüter, Arnulf-Dieter; et al. "Concerning the synthesis of [1.1.1]propellane". Chem. Ber. 122: 397–398. doi:10.1002/cber.19891220233.
- ↑ Mondanaro, Kathleen R.; Dailey, William P. "[1.1.1]Propellane". Org. Synth. 75: 98.; Coll. Vol., 10
- ↑ Kaszynski, Piotr; Michl, Josef (1988). "[n]Staffanes: a molecular-size "Tinkertoy" construction set for nanotechnology. Preparation of end-functionalized telomers and a polymer of [1.1.1]propellane". J. Am. Chem. Soc. 110 (15): 5225–5226. doi:10.1021/ja00223a070.