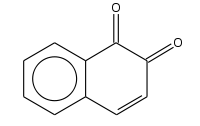
1,2-Naphthoquinone
 | |
| Names | |
|---|---|
| Other names
o-Naphthoquinone, β-naphthoquinone | |
| Identifiers | |
| 524-42-5 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL52347 |
| ChemSpider | 10217 |
| ECHA InfoCard | 100.007.602 |
| KEGG | C14783 |
| PubChem | 10667 |
| |
| |
| Properties | |
| C10H6O2 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2.
This double ketone (quinone) is a reactive metabolite of naphthalene and is found in diesel exhaust particles. The accumulation of this toxic metabolite in rats from doses of naphthalene has been shown to cause eye damage, including the formation of cataracts.[1]
See also
- 1,4-Naphthoquinone, an isomer of 1,2-naphthoquinone
- 2,6-Naphthoquinone, another isomer
References
- ↑ Qian, W.; Shichi, H. (2001). "Naphthoquinone-Induced Cataract in Mice: Possible Involvement of Ca2+ Release and Calpain Activation". Journal of Ocular Pharmacology and Therapeutics. 17 (4): 383–392. doi:10.1089/108076801753162799. PMID 11572469.
External links
- Troester, M. A.; Lindstrom, A. B.; Waidyanatha, S.; Kupper, L. L.; Rappaport, S. M. (2002). "Stability of Hemoglobin and Albumin Adducts of Naphthalene Oxide, 1,2-Naphthoquinone, and 1,4-Naphthoquinone" (pdf). Toxicological Sciences. 68 (2): 314–321. doi:10.1093/toxsci/68.2.314. PMID 12151627.
- Kikuno, S.; Taguchi, K.; Iwamoto, N.; et al. (2006). "1,2-Naphthoquinone Activates Vanilloid Receptor 1 through Increased Protein Tyrosine Phosphorylation, Leading to Contraction of Guinea Pig Trachea". Toxicology and Applied Pharmacology. 210 (1–2): 47–54. doi:10.1016/j.taap.2005.06.015. PMID 16039679.
This article is issued from Wikipedia - version of the 6/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.